- Written By
Sushmita Rout
- Last Modified 10-04-2025
Acid Rain: Causes, Effects
Acid rain isn’t pure acid falling from the sky; rather, rainfall or atmospheric moisture mixed with elements and gases cause the moisture to become more acidic than normal. Where pure water has a \(\text {pH}\) of \(7\), acid rain has a \(\text {pH}\) of about \(5.0-5.5\), and sometimes it could be \(4\) where there are a lot of industries and cars. It mainly occurs in the form of rain, snow, fog, and tiny bits of dry material. Erupting volcanoes, rotting vegetation release some chemicals that can cause acid rain, but most acid rain is a product of human activities. Let us explore more about acid rain in this article.
What is Acid Rain?
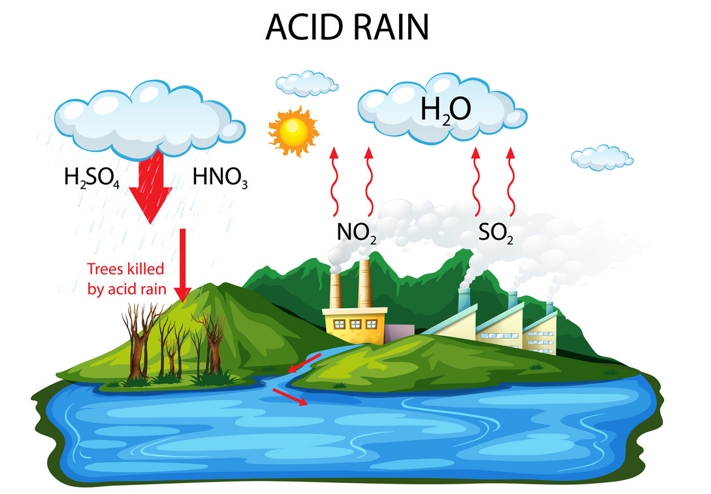
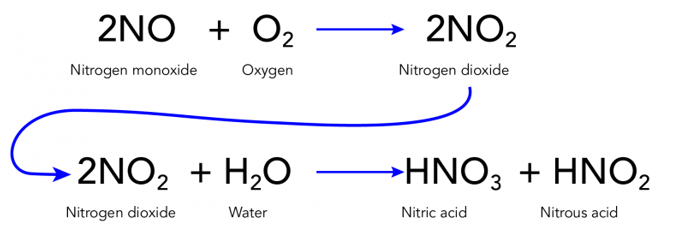
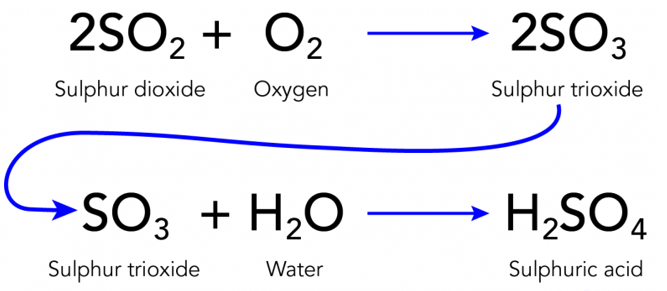
Acid rain, also known as acid deposition, is a broad term generally used to indicate any form of precipitation with acidic components such as sulfuric acid or nitric acid. These acids fall to the ground from the atmosphere in the form of rain, snow, fog or hail. It is a consequence of air pollution. When fuel is burnt, the smoke from a fire or the fumes from a car exhaust contains many invisible gases. These invisible gases are mainly nitrogen oxides and sulphur dioxide, which reacts with the tiny droplets of water in clouds to form sulphuric and nitric acids. These acid mixes with water droplets in the clouds and falls on earth’s surface as a very weak acid – which is why it is known as “acid rain”.
What Causes Acid Rain?
Emissions from fuels and vehicles are released into the air as pollutants. These pollutants react with the water, oxygen and other chemicals to form sulfuric and nitric acids transported to long distances. These acids fall to the earth as wet and dry deposition (dust, rain, snow, etc.). These depositions have harmful effects on soil, forests, streams, and lakes.
The major sources of \({\rm{S}}{{\rm{O}}_{\rm{2}}}\) and \({\rm{N}}{{\rm{O}}_{\rm{x}}}\) in the atmosphere are:
- Burning of fossil fuels to generate electricity. Two-thirds of \({\rm{S}}{{\rm{O}}_{\rm{2}}}\) and one-fourth of \({\rm{N}}{{\rm{O}}_{\rm{x}}}\) in the atmosphere comes from electric power generators.
- Exhaust from vehicles and heavy equipment.
- Emissions from oil refineries, manufacturing units and other industries.
Winds blow air laden with \({\rm{S}}{{\rm{O}}_{\rm{2}}}\) and \({\rm{N}}{{\rm{O}}_{\rm{x}}}\) over long distances and across borders making acid rain a global problem for everyone and not just those who live close to these sources.
How Acidic is Acid Rain?
Rain is always slightly acidic, with a \(\text {pH}\) value between \(5\) and \(6\). However, when it mixes with \({\rm{S}}{{\rm{O}}_{\rm{2}}}\) and \({\rm{N}}{{\rm{O}}_{\rm{x}}}\) the acidity can increase dramatically to a \(\text {pH}\) value of \(4\). Some rain has even been recorded as being \(\text {pH}\) value \(2\).
The heaviest recorded acid rain is only about as acidic as lemon juice or vinegar, and these don’t harm us – so why do we worry about acid rain? Let’s explore more about it.
Effects of Acid Rain
On Ecosystems
An ecosystem refers to geographical area that consists of a group of organisms and their physical environment, including air, water, and soil. All the components of an ecosystem are interdependent. If anyone species of plant or animal, or the soil or the water, is affected, it can impact everything else.
The data above illustrates the \(\text {pH}\) level at which many organisms may be lost if their environment becomes more acidic. Not all organisms can tolerate the same amount of acid.
On Fishes and Wildlife
Acid rain causes severe damage to the aquatic environments comprising streams, lakes, marshes, fishes and other wildlife. As the acidic rainwater flows through the soil, it leaches aluminium from clay particles and flows into streams and lakes.
At \(\text {pH} \,5\), most fish eggs cannot hatch, and some adult fishes die too. Even if a species of fish or animal tolerates acidic water, the animals that feed on them do not.
Lakes and Rivers
As acid rain ends up in water bodies, it causes an increase in the acidity of water bodies. Due to increased acidity, the water becomes low in mineral content, and fish and other water animals decline. Freshwater shrimps, mussels, snails are the most quickly affected by acidification, followed by fish such as salmon.
Acid rain increases the acidity of the water bodies and affects the aquatic species. It causes substances like aluminium to be washed off from the soil and released into water bodies, causing pollution. Henceforth, the entire aquatic ecosystem is hampered.
If a particular fish species disappear, the animals that feed on it will also disappear. If the extinct fish fed on a specific insect species, that insect population would grow; this, in turn, will cause the smaller insects or planktons to become extinct, on which the larger insect feeds. This is a symbiotic relationship of the ecosystem.
On Plants and Trees
Acid rain can affect plants and trees in several different ways. It-
- Dissolves and washes away the essential nutrients and minerals in the soil that help trees to grow.
- Leads to the release of harmful substances such as aluminium into the soil.
- Strips nutrients from the foliage of trees, leaving them with brown or dead leaves and needles.
- It wears away the waxy protective coating of leaves, damaging them and hampering their ability to photosynthesise properly.
All these effects damage the trees making them more vulnerable to be attacked by diseases, insects and by bad weather. It is not just trees that are affected by acid rain; organisms dependent on plants also suffer.
On Materials
Almost all type of material is affected by acid rain. It enhances the erosion process caused by the effects of the climate. Buildings, statues, vehicles, cables, pipes all suffer due to acid rain. The worst affected are the things made from limestone or sandstone. These types of rock are the most susceptible to be affected by air pollution and acid rain.
The consequences of this damage can be costly because:
- Damaged materials are needed to be repaired or replaced,
- High maintenance costs, and
- Loss of detail on stone and metal statues, monuments and tombstones.
On Human Health
Walking in acid rain or swimming in a lake polluted by acid rain can cause serious health issues, especially on the skin. When gases cause acid rain \({\rm{-S}}{{\rm{O}}_{\rm{2}}}\) and \({\rm{N}}{{\rm{O}}_{\rm{x}}}-\),-are in the air, it greatly affects the lung function leading to breathing difficulties and respiratory diseases.
What Can Be Done?
- Reduce emissions from burning fossil fuels – It is one of the cheapest ways to produce electricity and hence contributes a lot.
- Government initiatives to control pollution should be encouraged.
- Sulphur can be filtered from smoke by spraying a mixture of water and powdered limestone into the smokestack.
- Car exhausts should have catalytic converters that filter harmful gases released into the atmosphere.
a. Investment in alternative sources of energy.
b. The government should promote greater subsidies on opting for public transport to encourage people to use public transport rather than travelling by car.
- Prompting individual responsibility to save energy by switching off lights when not in use. When less electricity is used, pollution from power plants decreases.
- Walking, cycling and carpooling should be encouraged to reduce pollution from vehicles.
Summary of Acid Rain
Forests around the world are disappearing, and fishes are dying. There are dead lakes, which are crystal clear and contain no sign of living creatures in them. Fish-eating birds and animals are also threatened and are on the verge of extinction. Is acid rain responsible for all this? This page explains all of these. Acid rain affects countries and continents. It can precipitate in the form of snow, mists or even dry clouds of dust. Winds blow air laden with \({\rm{S}}{{\rm{O}}_{\rm{2}}}\) and \({\rm{N}}{{\rm{O}}_{\rm{x}}}\) over long distances and across borders making acid rain a global problem for everyone and not just those who live close to these sources.
FAQs
Q.1. What are the causes of acid rain?
Ans: Some of the causes of acid rain are-
1. Burning of fossil fuels to generate electricity. Two-thirds of \({\rm{S}}{{\rm{O}}_{\rm{2}}}\) and one-fourth of \({\rm{N}}{{\rm{O}}_{\rm{x}}}\) in the atmosphere come from electric power generators.
2. Exhaust from vehicles and heavy equipment.
3. Emissions from oil refineries, manufacturing units and other industries.
Q.2. What are the effects of acid rain?
Ans: The effects of acid rain are-
1. It causes severe damage to the aquatic environments comprising streams, lakes, marshes, fishes and other wildlife.
2. It leaches aluminium from clay particles and then flows into streams and lakes.
3. It dissolves and washes away the essential nutrients and minerals in the soil that help trees grow.
4. It leads to the release of harmful substances such as aluminium into the soil.
5. It strips nutrients from the foliage of trees, leaving them with brown or dead leaves and needles.
6. It wears away the waxy protective coating of leaves, damaging them and hampering their ability to photosynthesise properly
7. It enhances the erosion process caused by the effects of the climate. Buildings, statues, vehicles, cables, and pipes suffer from acid rain. The worst affected are the things made from limestone or sandstone.
Q.3. Why is acid rain harmful?
Ans: Acid rain and fog also damage forests, especially those at higher elevations. The acid deposits rob the soil of essential nutrients such as calcium and cause aluminium to be released, making it hard for trees to take water. Acids also harm trees’ leaves and needles.
Q.4. What are the ways to reduce acid rain?
Ans: 1. Reduce emissions from the burning of fossil fuels – It is one of the cheapest ways to produce electricity hence contributes a lot.
2. Government initiatives to control pollution should be encouraged.
3. Sulphur can be filtered from smoke by spraying a mixture of water and powdered limestone into the smokestack.
4. Car exhausts should have catalytic converters that filter harmful gases from being released into the atmosphere.
5. We need to invest in alternative sources of energy.
Q.5. Which gases cause acid rain?
Ans: Acid rain is caused by harmful gases such as sulphur dioxide and nitrogen dioxide in the air. These gases react with water to form sulphuric acid and nitric acid.
We hope this article on Acid Rain has helped you. If you have any queries, drop a comment below and we will get back to you.