- Written By
Ankita Sahay
- Last Modified 12-01-2025
Activation Energy Formula – Definition, S.I. Unit, Factors Affecting Activation Energy
Activation Energy Formula: In our body, millions of chemical reactions take place every time. These chemical reactions are responsible for supporting life. Now, many molecules exist in their stable states, and they need to break up to form new bonds as products. But this breaking and making of bonds need sufficient energy, which is known as “Activation Energy”. This activation energy can be defined as the minimum quantity of external energy required to convert the reactant into product. It is denoted as \({\text{‘}}{{\text{E}}_{\text{a}}}’\) having S.I. unit as kilojoules per mole \(\left( {{\text{kJ/mol}}} \right)\).
In simple language, we can say activation energy is a speed bump on the way of a reaction; the higher the speed bump, the lower will be the reaction speed and vice-versa. The reactant molecules must form an intermediate that will reach the activation energy followed by the formation of products. Without crossing this activation, energy reactants cannot form products. Thus, we can say that the rate of a chemical reaction is inversely proportional to the activation energy. Therefore, the greater value of activation energy leads to a slower rate of reaction. Various factors are involved in governing the rate of a reaction by altering the activation energy like reactant concentration, surface area, the physical state of the reactants, temperature, and catalyst. In this article, we will learn about the formula and various factors affecting activation energy in detail.
What is Activation Energy?
The term Activation Energy was introduced by the famous Swedish scientist Svante Arrhenius in \([1889]\).
In chemistry, Activation Energy is the minimum amount of energy that the reactant atoms or molecules require to reach a condition in which they can undergo chemical transformation or reaction to form the desired product.
Activation energy denoted as \(‘{{\text{E}}_{\text{a}}}’\) is measured in terms of joules per mole \(\left( {{\text{J/mol}}} \right)\), kilojoules per mole \(\left( {{\text{kJ/mol}}} \right)\) or kilocalories per mole \(\left( {{\text{kcal/mol}}} \right)\).
Before learning further about activation energy, we need to understand some terminologies:
- Threshold Energy – The minimum Kinetic Energy the molecules must possess to bring about effective collisions between two reactant molecules resulting in a successful chemical reaction between the two reactants, is known as Threshold Energy.
- Transition State – For the reaction to take place successfully, chemical bonds in the reactants need to be broken so that new bonds are formed to convert into products. To bring about such changes, the molecule must be deformed or contorted into an unstable state called the transition state. The transition state is a state of high energy, and reactant molecules need to achieve this state from the activation energy. Due to the instability of the transition state, molecules don’t stay there long but quickly proceed to the next step of the chemical reaction, i.e., the formation of products.
The activation energy is equal to the difference between the threshold energy needed for the reaction to occur and the average kinetic energy of all the reacting molecules in reactant species. i.e.,
\({{\rm{E}}_{\rm{a}}} = {{\rm{E}}_{{\rm{Threshold}}}}{\rm{ – }}{\mkern 1mu} {{\rm{E}}_{{\rm{average}}}}\)
This means if the activation energy for a reaction is low, the fractions of effective collisions are large, having high threshold energy, and the rate of a reaction is high. If the activation energy is high, the number of effective collisions is small, and the rate of the reaction is slow.
Thus, to conclude:
- Low Activation Energy – Faster reactions
- High Activation Energy – Slower reactions
Based on Arrhenius concept: Activation Energy is dependent on various factors; out of them, the temperature is the most important one. The Arrhenius equation deals with the quantitative basis of the relationship between the activation energy and the rate of a chemical reaction proceeds. The formula of Activation energy is given below:
\({\text{K = A}}{{\text{e}}^{\frac{{{\text{ – }}{{\text{E}}_{\text{a}}}}}{{{\text{RT}}}}}}\)
In the above equation, the notations are:
- \({\text{A}}\) as the pre-exponential factor
- T is the absolute temperature (Kelvin)
- k is the reaction rate coefficient
- \({{\text{E}}_{\text{a}}}\) is the Activation Energy
- \({\text{R}}\)is the Universal Gas constant
The Arrhenius equation is regarded as the best experimentally determined parameter that indicates the sensitivity of the reaction rate to temperature and activation energy.
Basically, activation energy is the potential barrier or energy barrier that the reactant molecules need to achieve to proceed in a chemical reaction.
Energy Changes during a Chemical Reaction
In a chemical reaction, either energy is released or absorbed. Based on these two factors, the chemical reactions are classified into two types:
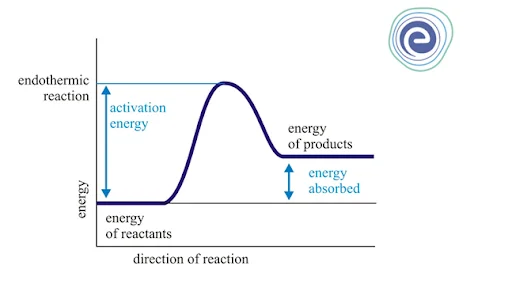
1. Endothermic Reactions
In an endothermic reaction, the energy is absorbed; thus, energy change will be positive. If \(\Delta {\rm{H}}\) is the enthalpy change of a chemical reaction, then:
\(\Delta {\rm{H}}{\mkern 1mu} {\rm{ = }}{\mkern 1mu} {\rm{ + ve}}\)
Because \(\Delta {\rm{H}}{\mkern 1mu} {\rm{ = }}{\mkern 1mu} \Delta {{\rm{H}}_{{\rm{Reactant}}{\mkern 1mu} }}{\rm{ – }}{\mkern 1mu} \Delta {{\rm{H}}_{{\rm{Product}}}}\)
And \(\Delta {\rm{H}}{\mkern 1mu} {\rm{ = }}{\mkern 1mu} \Delta {{\rm{H}}_{{\rm{Reactant}}{\mkern 1mu} }} > {\mkern 1mu} \Delta {{\rm{H}}_{{\rm{Product}}}}\)
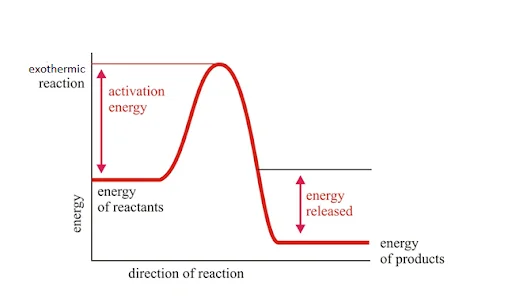
2. Exothermic Reactions
In an exothermic reaction, the energy is released; thus, energy change will be negative. If \(\Delta {\rm{H}}\) is the enthalpy change of a chemical reaction, then:
\(\Delta {\rm{H}}{\mkern 1mu} {\rm{ = }}{\mkern 1mu} – {\rm{ve}}\)
Because \(\Delta {\rm{H}}{\mkern 1mu} {\rm{ = }}{\mkern 1mu} \Delta {{\rm{H}}_{{\rm{Reactant}}{\mkern 1mu} }}{\rm{ – }}{\mkern 1mu} \Delta {{\rm{H}}_{{\rm{Product}}}}\)
And in this case: \(\Delta {{\rm{H}}_{{\rm{Reactant}}}}{\mkern 1mu} < {\mkern 1mu} \Delta {{\rm{H}}_{{\rm{Product}}}}\)
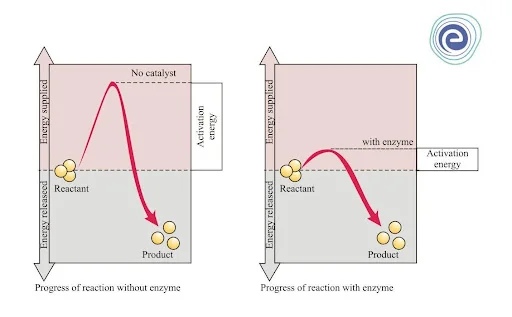
Effect of Catalyst on Activation Energy
Catalyst – A chemical substance that alters (either increases or decreases) the rate of a chemical reaction is known as a catalyst. This happens by altering the activation energy of a reaction. A catalyst lowers activation energy and increases the rate of a chemical reaction to a great extent. Apart from this, a catalyst does not bring any change in the reactants and the products.
Enzymes – Biological catalysts are known as enzymes.
Types of Catalysts
Based on the type of changes brought by a catalyst in a chemical reaction, a catalyst is classified into a positive catalyst and a negative catalyst. Let’s understand this based on the energy-profile diagrams.
Positive Catalyst
A catalyst that increases the rate of reaction by decreasing or lowering the activation energy by changing the path of the reaction is known as a positive catalyst.
Negative Catalyst (Reaction Inhibitor)
A catalyst that decreases or retards the rate of a chemical reaction by increasing the activation energy by taking a longer alternative pathway is known as a negative catalyst or inhibitor.
In a nutshell, Activation Energy is the minimum amount of energy required by a chemical reaction to occur. For example, combustion does not take place at room temperature; at least a spark is needed for the combustion to occur. Thus, activation energy is this ‘spark’, i.e., the minimum energy required for combustion. During a chemical reaction, firstly, reactants are excited to an intermediate form where the changes such as bond breaking and bond making occur. This energy level at which the intermediate product is formed is the activation energy. Lower the activation energy faster will be the conversion of intermediate to the products and vice versa.
This entire phenomenon is based on the collision theory, where an effective collision of molecules is needed for a reaction to occur. Thus, according to Arrhenius concept formula of Activation energy is \({\text{k = A}}{{\text{e}}^{\frac{{{\text{ – Ea}}}}{{{\text{RT}}}}}}\). Thus, we can infer that this Activation energy depends upon the temperature, surface area, the concentration of the reactants, and catalyst.
It is classified as an exothermic and endothermic reaction based on whether the energy is released or absorbed during a chemical reaction. Inside our body, there are various metabolic reactions that need to be completed very fast to help us stay alive. For this, catalysts or enzymes are used. They simply lower the activation energy and increase the rate of the reaction. Sometimes, inhibitors are also required, especially in the case of drugs; they elevate the activation energy and decrease the rate of the reaction. Thus, activation energy is very important in our life as every second we are dependent on various reactions both within our body as well as our surroundings.
Q.1. What is activation energy?
Ans. This activation energy can be defined as the minimum amount of external energy required to convert the reactant into the product in a chemical reaction. It is denoted as \(‘{{\text{E}}_{\text{a}}}’\) having S.I. unit as kilojoules per mole \(\left( {{\text{kJ/mol}}} \right)\).
Q.2. What is the activation energy in a chemical equation?
Ans. In chemistry, the activation energy is the minimum amount of energy that is required to activate atoms or molecules of the reactant to a condition in which they can undergo chemical reaction by effective collisions in the transition state of the chemical reaction. Lower the activation energy faster will be the conversion of intermediate to the products and vice versa. This entire phenomenon is based on the collision theory, where an effective collision of molecules is needed for a reaction to occur.
Q.3. Is activation energy positive or negative?
Ans. Activation energy is always positive. It is the energy supplied to the reactant molecules for their effective collisions to form the product. Since this energy is provided in a chemical reaction, it cannot be negative for elementary reactions. It may be negative for multi-step reactions in some cases where the reaction may be too fast to control.
Q.4. What does the activation energy depend on?
Ans. Various factors are involved in governing the rate of a reaction by altering the activation energy like reactant concentration, surface area, the physical state of the reactants, temperature, and catalyst. When the temperature is increased, the collision of molecules increases; hence, activation energy is achieved faster, and the reaction rate increases. Activation energy can be altered by the catalyst. A catalyst lowers activation energy and increases the rate of a chemical reaction to a great extent. Apart from this, a catalyst does not bring any change in the reactants and the products. The same work is done by the enzymes as they are biocatalysts.
Q.5. What are the types of catalyst?
Ans. Based on the type of changes brought by a catalyst in a chemical reaction, a catalyst is classified into a positive catalyst and a negative catalyst.
We hope this article on Activation Energy Formula has helped you. If you have any queries, drop a comment below, and we will get back to you.