- Written By
Sushmita Rout
- Last Modified 31-12-2024
Amorphous Forms of Carbon
Amorphous Forms of Carbon: Carbon is an interesting element. The atoms of carbon can be arranged to form one of the hardest known materials in the world-diamond as well as soft and pliable graphite.
Carbon is recognised as the ‘King of the Elements’ in the periodic table and is the primary ingredient for most life forms on the Earth. From food, books, clothes, medicines are all based on this versatile element carbon. Besides, all living structures are carbon-based. Let’s learn about its amorphous forms.
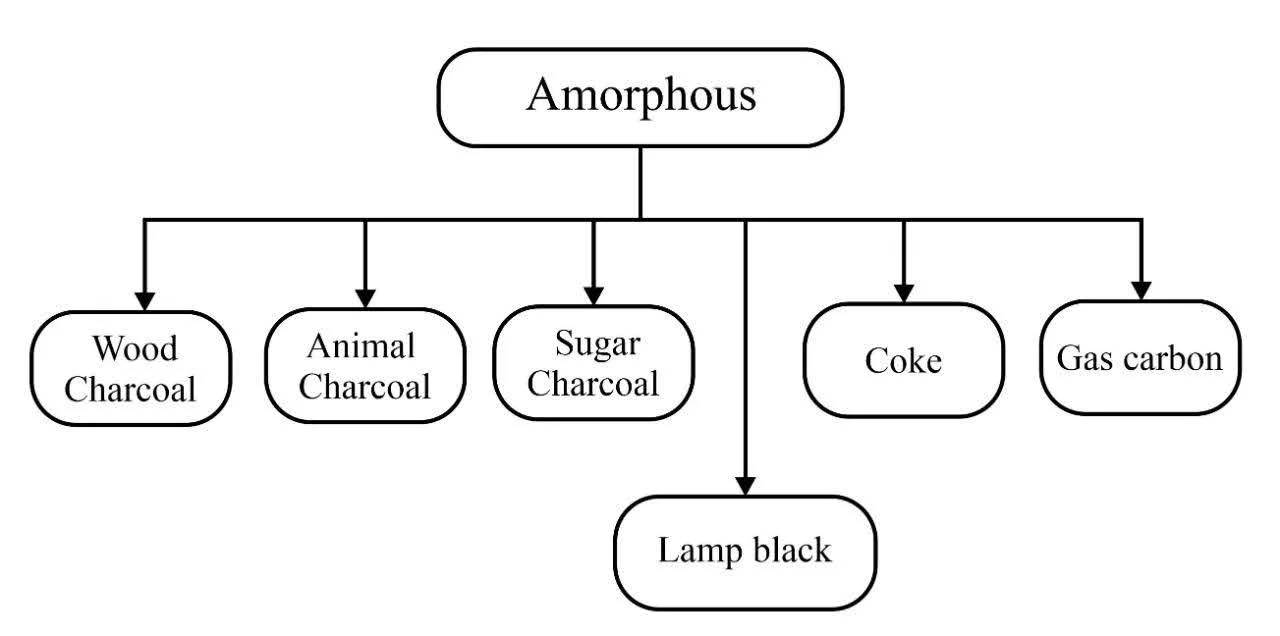
Amorphous solids are solids that lack a long-range, three-dimensional arrangement of atoms. They exhibit short-range order and a more random arrangement of molecules. The amorphous forms of carbon are obtained when wood, bones, kerosene oil etc., are heated in the absence of air. Some of the amorphous forms of carbon are listed below-
Charcoal
Charcoal is obtained when a carbon-based compound is heated in the absence or limited supply of air. Such a process is called destructive distillation.
Based on its source, charcoal is classified into three types. These are-
1. Wood charcoal: As the name suggests, wood charcoal is obtained by heating wood in the absence of air. This process is also known as the destructive distillation of wood which leaves behind a black mass. It is called wood charcoal, i.e. the residue of sawdust.
Properties and uses of wood charcoal:
- It is a porous, black and brittle solid.
- It floats on water as it can hold air in its pores.
- It is a good adsorbent and a bad conductor of electricity.
- It is used as a fuel and is an essential constituent of gunpowder.
- It is used in respirators or gas masks as it adsorbs harmful gases.
- It adsorbs the poisonous vapours of nicotine, hence is used as a cigarette filter.
- It helps in refining oils and fats and decolourising sugar syrup.
- It is used as a disinfectant.
Animal Charcoal
As the name suggests, animal charcoal is obtained by heating bones in the absence of air. This process is also known as the destructive distillation of bones in the absence of air. Animal charcoal is also known as bone charcoal. The primary constituent of animal charcoal is calcium phosphate, with \(10 – 12\% \) of carbon content.
Properties and Uses of Animal Charcoal
- It can extract coloured materials from solutions.
- Liquids can easily wet it.
- It helps in decolourising sugar solutions.
- It is used in the manufacture of phosphorus and its compounds.
Sugar Charcoal
It is the purest form of amorphous carbon and is prepared by destructive distillation of cane sugar or glucose in the absence of air.
\(\mathop {{{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}{{\text{O}}_{\text{6}}}}\limits_{{\text{Glucose}}} \xrightarrow{{{\text{heat}}}}\mathop {{\text{6C}}}\limits_{{\text{Carbon}}} + \mathop {6{{\text{H}}_2}{\text{O}}}\limits_{{\text{Water}}} \)
\(\mathop {{{\text{C}}_{12}}{{\text{H}}_{{\text{22}}}}{{\text{O}}_{11}}}\limits_{{\text{Glucose}}} \xrightarrow{{{\text{heat}}}}\mathop {{\text{12C}}}\limits_{{\text{Carbon}}} + \mathop {{\text{11}}{{\text{H}}_2}{\text{O}}}\limits_{{\text{Water}}} \)
It is also obtained by the dehydration of glucose or cane sugar in the presence of concentrated sulphuric acid. The acid acts as the dehydrating agent and absorbs the water to leave behind carbon.
Coal
Coal is a black material made up of vegetable origin. It is a mixture of compounds of carbon, hydrogen, oxygen. It is formed by carbonisation under the surface of Earth.
Coal was formed in prehistoric times, around \(200\) million to \(250\) million years ago. Plants and animals remain got buried under the surface of Earth, and due to hot and humid conditions, hydrogen and oxygen were removed, leaving behind carbon. Due to the extreme pressure of Earth, the carbon compacts to form coal.
Based on the percentage of carbon, there are different varieties of coal. These are –
- Peat \(60\%\)
- Lignite (soft coal) \(67\%\)
- Bituminous (household coal) \(88\%\)
- Anthracite (hard coal) \(96\%\)
Anthracite is the most superior, whereas peat is the most inferior quality of coal. Coal is a major source of fuel and is used to make coke. Coke is the purest form of coal.
Uses of Coal
- It is used as a source of fuel in factories and thermal power stations.
- The destructive distillation of coal yields products like coal tar, coke, coal gas, ammonia liquor, and coal gas that are extensively used for domestic and industrial purposes.
Coke
Preparation
Destructive distillation of bituminous coal at \(1300^\circ {\rm{C}}\) results in a mixture of liquids and gases.
- Gaseous products mainly comprise coal gas
- The liquid product is made up of ammoniacal liquor and coal tar. Ammoniacal liquor forms the upper layer, whereas coal tar forms the lower layer.
- A solid residue of coke (\(85\%\) carbon) is also obtained.
Coke is of two types, hard coke and soft coke.
(i) Hard coke: It is a light, lustrous substance that is used in industrial and metallurgical furnaces.
(ii) Soft Coke: It is a black and porous substance that is widely used in household furnaces.
Uses of Coke
- It is used as a smokeless fuel.
- It acts as a reducing agent in the production of iron and steel.
- In the manufacture of producer gas \(\left( {{\rm{CO}} + {{\rm{N}}_2}} \right)\), water gas \(\left( {{\rm{CO}} + {{\rm{H}}_2}} \right)\), and artificial graphite.
Lamp Black
Lamp Black is made up of \(99\) per cent carbon. It is obtained by heating carbon-rich substances like kerosene, petroleum, turpentine oil etc., in a limited supply of air. The soot is collected over damp blankets in chambers from where it is obtained by jerking.
Uses of Lamp Black
- It is used in making printing ink.
- As a filler in tyres to increase their durability.
- It is used in kajal and shoe polish.
Summary
The Earth’s crust is made up of only \(0.02\%\) carbon. Such a meagre amount of carbon is available in the form of minerals like coal, petroleum, carbonates, hydrogen carbonates. The atmosphere has \(0.03\%\) of carbon dioxide. In spite of such a small amount of carbon available in nature, the importance of carbon seems to be immense. In this article, we learnt the amorphous forms of carbon. We also learnt the properties and uses of these compounds.
Frequently Asked Questions
Q.1. What do you mean by allotropes?
Ans: Allotrope refers to the chemical element that exists in one or more physical forms but in the same physical state. Allotropes of a chemical element may show differences in chemical and physical properties. Such as graphite and diamond.
Q.2. What are the amorphous allotropes of carbon?
Ans: The amorphous form of carbon is- coal, coke, lampblack, wood charcoal.
Q.3. How many types of coal are there?
Ans: Based on the percentage of carbon, there are different varieties of coal. These are – Peat \(60\%)\), Lignite or soft coal \((67\%)\), bituminous or household coal \((88\%)\), anthracite or hard coal \((96\%)\).
Q.4. What are the four forms of carbon?
Ans: The four forms of carbon include graphite, diamond, amorphous carbon and fullerenes.
Q.5. Which is the purest form of amorphous carbon?
Ans: Sugar charcoal is obtained from the carbonisation of sugar is the purest form of amorphous carbon.
We hope this article on the Amorphous Forms of Carbon has helped you. If you have any queries, drop a comment below, and we will get back to you.