- Written By
Akanksha P John
- Last Modified 27-01-2025
Chemical Properties of Group 17 Elements: Introduction, Properties, Uses
Chemical Properties of Group \(17\) Elements: A chemical property describes a substance’s ability to undergo a specific chemical change. However, unlike physical properties, chemical properties can only be observed while the substance is being transformed into another substance.
The elements in the \({\text{s}}\) and \({\text{p}}\) blocks of the periodic table are known as representative elements or main group elements. Elements in groups \(1\) and \(2\) are classified as \({\text{s}}\)-block elements, while elements in groups \(13\) to \(18\) are classified as \({\text{p}}\)-block elements.
Fluorine is a highly electronegative element that belongs to the group of halogens. Many other elements, such as fluorine, have general properties. In this article, you will learn everything there is to know about the chemical properties of group \(17\) in the \({\text{p}}\)-block, also known as Halogens.
Group 17 Elements
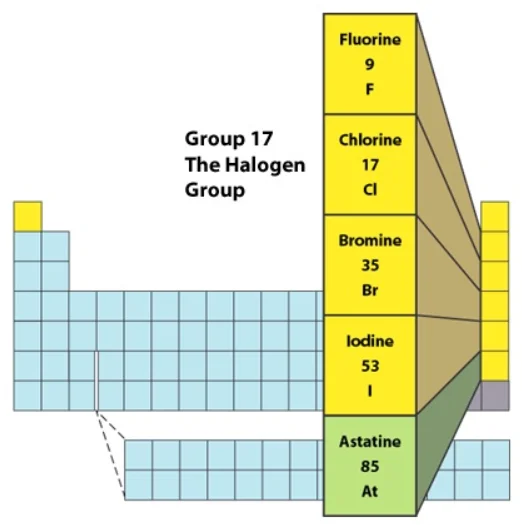
Group \(17\) is included in the \({\text{p}}\)-block of the periodic table and consists of five elements: fluorine \(\left({\text{F}} \right),\) chlorine \(\left({{\text{Cl}}} \right),\) bromine \(\left({{\text{Br}}} \right),\) iodine \(\left({\text{I}} \right),\) and astatine \(\left({{\text{At}}} \right).\)
This class of elements is commonly referred to as halogens. Halogens are derived from two Greek words: halo, which means sea salt, and gens, which means the product, as in sea salt products. This is due to the fact that the first three members exist as salts in seawater. These are some of the nonmetal elements that are the most reactive. The family’s final member, astatine, is a radioactive element.
Occurrence of Group 17 Elements
Because halogens are highly reactive in nature, they do not exist in their state. Except for astatine, all other elements are abundant as halide ions in the earth’s crust.
Halogens exist in all three states of matter.
Fluorine is the \({13^{{\text{th}}}}\) most abundant element in the earth’s crust in terms of weight. It exists in the gaseous state at room temperature. Insoluble fluorides, cryolites, fluorspar, and fluorapatite are the most common forms. Fluorine can also be found in soil, plants, stream water, animal bones and teeth, and water.
By weight, chlorine is the \({20^{{\text{th}}}}\) most abundant element in the earth’s crust. It exists in the gaseous state at room temperature. The ocean contains \(1.5\% \) sodium chloride by weight.
In ocean water, chlorine, bromine, and iodine can be found as chlorides, bromides, and iodides of highly reactive metals such as sodium, potassium, magnesium, and calcium.
Iodides are found in trace amounts in ocean water. Iodine is primarily obtained from seaweeds and crude chile saltpetre.
At room temperature, astatine is a naturally occurring radioactive element that exists as a solid.
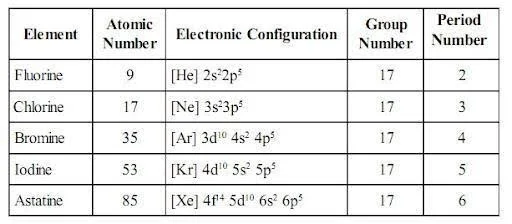
Electronic Configuration of Group 17 Elements
The elements in Group \(17\) have \(7\) electrons in their valence shell and have the following general electronic configuration: \({\text{n}}{{\text{s}}^2}{\text{n}}{{\text{p}}^5}.\)
Chemical Characteristics of Group 17 Elements
Halides are formed when halogens react with metals and nonmetals. Of all the halogens, fluorine is the most reactive. The reactivity of the halogens decreases as one moves down the group.
The high reactivity of halogens is due to the following reasons:
- i. Low bond dissociation enthalpies
- ii. High electron gain enthalpy
Oxidising Power of Group 17 Elements
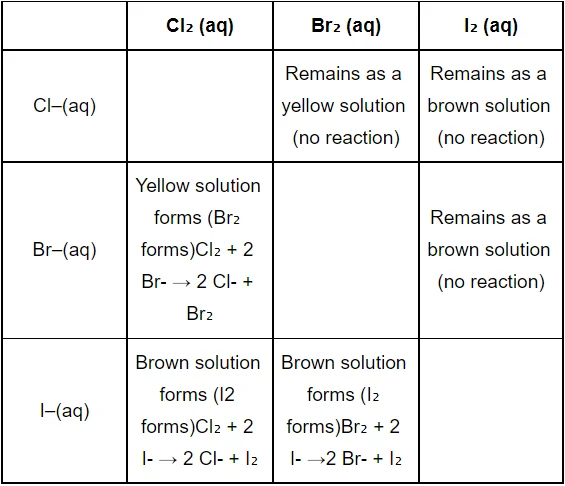
Since all halogens have a strong tendency to accept electrons, they are excellent oxidising agents. Fluorine is the most grounded oxidising agent among the numerous halogens, capable of converting all other halide particles to halogen in a solution. The oxidising power decreases as we move down the group from \({\text{F}}\) to \({\text{I}}.\) Chlorine also can oxidise bromide particles to bromine and iodide particles to iodine.
\({\text{C}}{{\text{l}}_2} + 2{\text{B}}{{\text{r}}^ – } \to {\text{B}}{{\text{r}}_2} + 2{\text{C}}{{\text{l}}^ – }\)
\({\text{C}}{{\text{l}}_2} + 2{{\text{I}}^ – } \to {{\text{I}}_2} + 2{\text{C}}{{\text{l}}^ – }\)
Similarly, bromine can oxidise iodide particles to iodine. \({\text{B}}{{\text{r}}_2} + 2{{\text{I}}^ – } \to {{\text{I}}_2} + 2{\text{B}}{{\text{r}}^ – }\)
Halide particles act as reducing agents as well. Their reducing capacity increases as they progress from fluoride to iodide particles.
Reaction with Hydrogen
To form acidic hydrogen halides, all halogens react with hydrogen. From \({\text{HF}}\) to \({\text{HI}},\) the acidity of these hydrogen halides increases. Regardless, the reactivity of halogens towards hydrogen decreases as one progresses from fluorine to iodine. Fluorine reacts violently in the dark; chlorine requires sunlight; and bromine reacts with hydrogen only when heated. When heated in the presence of platinum as a catalyst, iodine reacts with hydrogen.
In dark-\({{\text{H}}_2}{{\text{F}}_2} \to 2{\text{HF}}\)
In sunlight-\({{\text{H}}_2} + {\text{C}}{{\text{l}}_2} \to 2{\text{HCl}}\)
On heating-\({{\text{H}}_2} + {\text{B}}{{\text{r}}_2} \to 2{\text{HBr}}\)
In the presence of catalyst- \({{\text{H}}_2} + {{\text{I}}_2} \to 2{\text{HI}}\)
Reaction with Oxygen
Halogens, like other elements, form oxides with oxygen in the same way. However, the majority of halogen oxides are not stable. In addition to oxides, halogens form halogen oxoacids and oxo-anions. The general formula for oxides ranges from \({{\text{X}}_2}{\text{O}}\) to \({{\text{X}}_2}{{\text{O}}_7},\) while the general formula for oxyacids ranges from \({\text{HO}} – {\text{X}}\) to \({\text{HO}} – {\text{X}}{{\text{O}}_3}\) and for oxo-anions ranges from \({\text{X}}{{\text{O}}^ – }\) to \({\text{X}}{{\text{O}}_4}^ – .\)
The stability of oxides formed by halogens decreases in the order-
\({\text{I}} > {\text{Cl}} > {\text{Br}}\)
Since halogens have high reactivity, they instantly react with the majority of metals to form metal halides.
Sodium, for example, reacts with chlorine gas to form sodium chloride. The production of sodium chloride is an exothermic reaction that results in a splendid yellow light with a lot of heat energy.
\(2{\text{Na}}\left({\text{s}} \right) + {\text{C}}{{\text{l}}_2}\left({\text{g}} \right) \to 2{\text{NaCl}}\left({\text{s}} \right)\)
Bromine reacts with magnesium to give magnesium bromide.
\({\text{Mg}}\left({\text{s}} \right) + {\text{B}}{{\text{r}}_2}\left({\text{g}} \right) \to {\text{MgB}}{{\text{r}}_2}\left({\text{s}} \right)\)
Because of the high electronegativity of halogen and the high electropositivity of metals, metal halides are ionic in nature. From fluorine to iodine, the ionic character of metal halides decreases, and the order is as follows:
\({\text{MF}} > {\text{MCl}} > {\text{MBr}} > {\text{MI}}\)
Reaction with other Halogens
Interhalogen compounds are formed when halogens react with one another. The general formula for these compounds is \({\text{X}}{{\text{Y}}_{\text{n}}},\) where \({\text{n}}\) is one, three, five, or seven. \(”{\text{X}}”\) in a given equation must be the less electronegative halogen as compared to \(”{\text{Y}}”.\)
Anomalous Behaviour of Fluorine
Because of its small nuclear size, high electronegativity, low bond dissociation energy, and lack of accessibility of d-orbitals, fluorine’s valence shell exhibit anomalous behaviour in properties such as ionisation energy, bond dissociation energy, electronegativity, electrode potentials, ionic and covalent radii, electron gain enthalpy, melting point, and boiling point.
Uses of Halogens
Some of the applications of halogens are as follows-
Fluorine Uses
- i. Fluorine is present in drinking water and toothpaste because it aids in the prevention of tooth decay.
- ii. It can be found in ceramic clay.
- iii. They can be found in chlorofluorocarbons, which are used as refrigerants.
- iv. They are used to produce nuclear energy.
Chlorine Uses
- i. Chlorine is used to purify drinking water and swimming pools.
- ii. It can be found in PVC (wire insulation).
- iii. It is used to sterilise hospital equipment.
- iv. It is also a component of certain pesticides such as DDT (dichlorodiphenyltrichloroethane).
Bromine Uses
- i. Bromine has fire retardant properties, so it is used as a fire extinguisher.
- ii. It is employed in the treatment of pneumonia and Alzheimer’s disease.
- iii. Methyl bromide is a pesticide that is used to control the spread of bacteria while also allowing crop storage.
Iodine Uses
- i. Iodine plays an important role in the functioning of our body’s thyroid gland.
- ii. Solutions used to clean open wounds contain iodine.
- iii. In photography, silver iodide is used.
Astatine Uses
- i. The element astatine is radioactive.
- ii. It has aided in cancer research.
Summary of Group 17 Elements
In this article, we studied that halogens have a general electronic configuration \({\text{n}}{{\text{s}}^2}{\text{n}}{{\text{p}}^5}.\) Now we know some of the uses of each element of group \(17.\) We also studied the following points:
- i. Halogens are strong oxidizing agents.
- ii. Halogens form acidic hydrogen halides and their acidic strength increases from fluorine to iodine.
- iii. Halogens form oxides that range from \({{\text{X}}_2}{\text{O}}\) to \({{\text{X}}_2}{{\text{O}}_7}.\)
- iv. Halogens form metal halides, which are ionic in nature.
- v.Halogens react with other halogens as well, and they form interhalogen compounds having a general formula \({\text{X}}{{\text{Y}}_{\text{n}}},\) where \({\text{n}} = 1,3,5\) or \(7.\)
MCQs of Group 17 Elements
- Which halogen will give the best yield of a single monohalogenation product?
a.) fluorine
b.) chlorine
c.) Bromine
d.) Iodine
Correct Answer- a
Hint- This element is most electronegative in nature.
- Which of the following is the strongest oxidizing agent in the halogen family?
a.) chlorine
b.) fluorine
c.) astatine
d.) Iodine
Correct Answer- b
Hint- This element is most electronegative in nature.
- HF is not kept inside-
a.) Plastic bottle
b.) Glass bottle
c.) Tin bottle
d.) Iron bottle
Correct Answer- b
Hint- HF is weakly acidic in nature.
- Which of the following has the strongest bond?
a.) F-F
b.) F-Br
c.) Cl-Br
d.) F-Cl
Correct Answer- d
Hint- This has the highest bond energy among all the others.
- Fluorine shows anomalous behaviour in group 17 due to-
a.) its small size
b.) high electronegativity
c.) absence of d-orbitals
d.) All of the above
Correct Answer- d
Hint- The first element of every group shows unique behaviour.
- Fluorine does not show positive oxidation state because-
a.) It is most electronegative element
b.) It forms only anions in ionic compounds
c.) It cannot form multiple bonds
d.) It shows non-bonded electron pair repulsion due to small size
Correct Answer- a
Hint- oxidation state is decided based on electronegativity.
- The element which can displace three other halogens from their compounds is-
a.) Cl
b.) Br
c.) I
d.) F
Correct Answer- d
Hint- It is a strong oxidizing agent.
- The group which is called halogen family is-
a.) Group 16
b.) Group 17
c.) Group 10
d.) Group 18
Correct Answer- b
Hint- Elements in this group have seven valence electrons.
- In NaOX, X cannot be-
a.) fluorine
b.) chlorine
c.) Bromine
d.) Iodine
Correct Answer- d
Hint- This element plays an important role in the functioning of our body’s thyroid gland.
Halogen elements are those which are-
a.) diatomic and form X– ions
b.) monoatomic and form X- ions
c.) diatomic and form X- ions
d.) monoatomic and form X– ions
Correct Answer- c
Hint- Halogens need one electron to complete their octet.
FAQs on Group 17 Elements
Q.1. What are the chemical properties of group 17?
Ans: The chemical properties of halogens are as follows:
i. Halogens are strong oxidizing agents.
ii. Halogens form acidic hydrogen halides and their acidic strength increases from fluorine to iodine.
iii. Halogens form oxides that range from \({{\text{X}}_2}{\text{O}}\) to \({{\text{X}}_2}{{\text{O}}_7}.\)
iv. Halogens form metal halides, which are ionic in nature.
v. Halogens react with other halogens as well, and they form interhalogen compounds having a general formula \({\text{X}}{{\text{Y}}_{\text{n}}},\) where \({\text{n}} = 1,3,5\) or \(7.\)
Q.2. Does group 17 have similar chemical properties?
Ans: All halogens have chemical properties that are similar. This is due to the fact that all halogen atoms have seven valence electrons.
Q.3. What is special about group 17 on the periodic table?
Ans: On the periodic table, the halogens are to the left of the noble gases. Because halogen elements have seven valence electrons, they only need one more electron to form a complete octet. Because of this, they are more reactive than other nonmetal groups.
Q.4. Why does fluorine always have an oxidation state of –1 in its compounds?
Ans: Electronegativity begins to rise across a period and decreases as one moves down the group. Fluorine has the highest electronegativity of any element because fluorine has seven valence electrons, and it requires only one more electron (eight valence electrons) to complete its octet. As a result, it will be more likely to pull an electron from a nearby atom.
Q.5. Why are halogens strong oxidizing agents?
Ans: Halogens need only one electron to complete their octet and to attain the stable noble gas configuration. Also, halogens are highly electronegative with low bond dissociation energies and high negative electron gain enthalpies. Therefore, they have a high tendency to gain an electron. Hence, they act as a strong oxidizing agent.