- Written By
Paramjit Singh
- Last Modified 30-03-2025
Dinitrogen: Preparation, Structure, Properties and Uses
Dinitrogen: Nitrogen is a colourless, odourless, and tasteless element that is plentiful in nature. Daniel Rutherford, a Scottish physician, was the first to discover it in \(1772\). The human body is made up of \(3\% \) nitrogen. The element nitrogen is the \({{\rm{7}}^{{\rm{th}}}}\) most prevalent in the universe. At ambient temperature, nitrogen, which makes up around \({\rm{78\% }}\) of our atmosphere, is a colourless, odourless, tasteless, and chemically inert gas. It gets its name from the Greek words nitron and genes, which mean “to make soda.” During the \({\rm{1500 s}}\) and \({\rm{1600 s}}\), scientists speculated that there was a third gas in the atmosphere, in addition to carbon dioxide and oxygen.
Daniel Rutherford (and others independently, such as Priestly and Cavendish) discovered it in \(1772\) when he was able to remove oxygen and carbon dioxide from a confined tube full of air. He demonstrated that there was residual gas that could not be burned, such as oxygen or carbon dioxide. While his experiment demonstrated the existence of nitrogen, other tests were taking place in London, where the substance was referred to as “burnt” or “dephlogisticated air.”
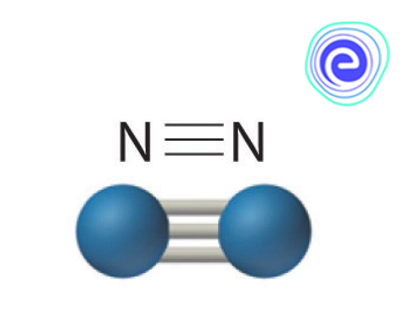
Structure of Dinitrogen
Because of the strong triple bond that holds the \({\rm{N’s}}\) together, \({{\rm{N}}_{\rm{2}}}\) is chemically inert. Dinitrogen is a form of nitrogen. \({{\rm{N}}_{\rm{2}}}\) is a gas (bp \({\rm{77}}{\rm{.3}}\,{\rm{K}}\)). \({{\rm{N}}_{\rm{2}}}\) makes up \({\rm{78\% }}\) of the atmosphere. \({{\rm{N}}_{\rm{2}}}{\rm{‘s}}\) inert behaviour is attributed to the \({\rm{N}} \equiv {\rm{N}}\) triple bond. Because of its tiny size, it forms strong \({\rm{p – p}}\) multiple bonds. \({{\rm{N}}_2}\) is a strong bonding gas with a small internuclear distance \(\left( {{\rm{1}}{\rm{.094}}\,\mathop {\rm{A}}\limits^{\rm{o}} } \right)\) and the ability to create up to four bonds.
Isotopes
Chemical exchanges or thermal diffusion can separate the two naturally occurring isotopes of nitrogen, nitrogen-\(14\) and nitrogen-\(15\). Nitrogen has isotopes with mass numbers of \(12, 13, 16\), and \(17\), but they are radioactive and unstable. Nitrogen \(14\) is the most abundant nitrogen isotope, accounting for more than \({\rm{99\% }}\) of all nitrogen on the planet. It is a non-radioactive, stable chemical element. Nitrogen-\(14\) is the most widely used element, with applications in agriculture, food preservation, biochemicals, and biological research. In the atmosphere and among many living species, nitrogen-\(14\) is abundant.
It possesses \(5\) valence electrons and is a poor conductor of electricity. The other stable form of nitrogen is nitrogen-\(15\). It’s frequently used in medical research and storage. Because the element is non-radioactive, it can be employed in agricultural activities on occasion. Nitrogen-\(15\) is also employed in brain research, particularly nuclear magnetic resonance spectroscopy (NMR), since, unlike nitrogen-\(14\) (which has a nuclear spin of \(1\)), it has a nuclear spin of \(1/2\), which helps with MRI and NMR studies. Finally, in biology, nitrogen-\(15\) can be utilised as a marker or in some proteins. Scientists mostly employ this substance for research purposes, and its full potential for application in brain research has yet to be discovered.
Preparation of Dinitrogen
- Dinitrogen is made in the lab by reacting sodium nitrite with an aqueous solution of ammonium chloride.
\({\rm{N}}{{\rm{H}}_{\rm{4}}}{\rm{Cl}}\left( {{\rm{aq}}{\rm{.}}} \right){\rm{ + NaN}}{{\rm{O}}_{\rm{2}}}\left( {{\rm{aq}}{\rm{.}}} \right) \to {{\rm{N}}_{\rm{2}}}\left( {\rm{g}} \right){\rm{ + 2}}{{\rm{H}}_{\rm{2}}}{\rm{O}}\left( {\rm{l}} \right){\rm{ + NaCl}}\left( {{\rm{a}}{\rm{.q}}{\rm{.}}} \right)\)
- It can be obtained by the thermal decomposition of ammonium dichromate.
\({\left( {{\rm{N}}{{\rm{H}}_{\rm{4}}}} \right)_{\rm{2}}}{\rm{C}}{{\rm{r}}_{\rm{2}}}{{\rm{O}}_{\rm{7}}}\left( {{\rm{Heat}}} \right) \to {{\rm{N}}_{\rm{2}}}\left( {\rm{g}} \right){\rm{ + 4}}{{\rm{H}}_{\rm{2}}}{\rm{O}}\left( {\rm{I}} \right){\rm{ + C}}{{\rm{r}}_{\rm{2}}}{{\rm{O}}_{\rm{3}}}\left( {\rm{s}} \right)\)
- The thermal decomposition of sodium or barium azide yields very pure nitrogen.
\({\rm{Ba}}{\left( {{{\rm{N}}_{\rm{3}}}} \right)_{\rm{2}}} \to {\rm{Ba + 3}}{{\rm{H}}_{\rm{2}}}\)
- Commercial nitrogen can be obtained through liquefaction and fractional distillation of air. There are two main steps in this procedure:
Step 1
By applying a high pressure of \(100\) to \(200\) atmospheres, we may convert air to liquid air. Following that, we feed the compressed air through a fine jet, where it expands. This technique is repeated multiple times, resulting in the production of liquid air.
Step 2
Fractional distillation is applied to the resulting liquid. Dinitrogen has a lower boiling point than liquid oxygen; therefore, it separates, leaving liquid oxygen behind. The impure liquid provides us with nitrogen.
Impurities such as \({\rm{NO}}\) and \({\rm{HN}}{{\rm{O}}_{\rm{3}}}\) are present in the products, which can be eliminated by the thermal decomposition of ammonium dichromate. Another way to eliminate contaminants is to run the gaseous mixture through a sulphuric acid solution containing potassium dichromate.
\({\left( {{\rm{N}}{{\rm{H}}_{\rm{4}}}} \right)_{\rm{2}}}{\rm{C}}{{\rm{r}}_{\rm{2}}}{{\rm{O}}_{\rm{7}}} \to {{\rm{N}}_{\rm{2}}}{\rm{ + 4}}{{\rm{H}}_{\rm{4}}}{\rm{O + C}}{{\rm{r}}_{\rm{2}}}{{\rm{O}}_{\rm{3}}}\)
- Nitrogen can also be produced by the reduction of air with carbon and by oxidation of ammonia with oxygen gas.
\({\rm{Air}}\left( {{\rm{4}}{{\rm{N}}_{\rm{2}}}{\rm{ + }}{{\rm{O}}_{\rm{2}}}} \right){\rm{ + C}} \to {\rm{4}}{{\rm{N}}_2} + {\rm{C}}{{\rm{O}}_{\rm{2}}}\)
\({\rm{4N}}{{\rm{H}}_{\rm{3}}}{\rm{ + 3}}{{\rm{O}}_{\rm{2}}} \to 2{{\rm{N}}_{\rm{2}}}{\rm{ + 6}}{{\rm{H}}_{\rm{2}}}{\rm{O}}\)
\({\rm{2N}}{{\rm{H}}_{\rm{3}}}{\rm{ + 3C}}{{\rm{l}}_{\rm{2}}} \to {{\rm{N}}_{\rm{2}}}{\rm{ + 6HCl}}\)
Physical Properties of Dinitrogen
Now we’ll look into dinitrogen’s physical properties.
1. In nature, nitrogen is colourless, odourless, and diamagnetic.
2. Nitrogen is a non-toxic gas that is only sparingly soluble in water and condenses to create a colourless liquid. This, on solidification, results in the formation of snow like mass.
Chemical Properties of Dinitrogen
- At normal temperatures, \({{\rm{N}}_{\rm{2}}}\) is practically non-reactive. It doesn’t burn and doesn’t enable combustion. The chemical inertness of \({{\rm{N}}_{\rm{2}}}\) at normal temperatures is related to the molecule’s strong stability due to the triple bond.
- The two nitrogen atoms in a molecule of \({{\rm{N}}_{\rm{2}}}\) are connected by a triple bond. The bond enthalpy (the amount of heat energy required to break a chemical bond) of the triple bond is extremely high. \({{\rm{N}}_{\rm{2}}}\) is almost unreactive with most reagents due to its extremely high bond dissociation enthalpy.
- It does, however, interact with some metals and non-metals at high temperatures to generate nitrides, which are ionic and covalent compounds.
- Because of the \({\rm{N}} \equiv {\rm{N}}\) bond, dinitrogen has a high bond enthalpy. It is inert at room temperature as a result of this. However, as the temperature rises, the reactivity goes up with it. Nitrogen molecules react with metals at high temperatures. The relevant ionic nitrides are formed as a result of this reaction. Covalent nitrides are formed when the molecules react with non-metals.
- At about \({\rm{773}}\,{\rm{K}}\), it reacts with hydrogen to form ammonia in Haber Process.
- At a temperature of \(2000\,{\rm{K}}\), the interaction of a nitrogen molecule with an oxygen molecule produces nitric oxide.
Uses of Dinitrogen
The Haber process, which produces ammonia, is the most common commercial application for elemental nitrogen gas. The Haber process produces ammonia, which is usually used to make fertilisers like urea. In the iron and steel industries, for example, nitrogen is employed in chemical reactions that require an inert environment. Liquid nitrogen is also utilised in cryosurgery and as a refrigerant. Nitrogen-rich foods have a longer shelf life and aid to suppress bacterial growth. Some MRI machines and other temperature control systems employ liquid nitrogen as a coolant. Nitrous oxide, popularly known as laughing gas, is a dental anaesthetic and is used in other topical surgical procedures for anaesthetic purposes.
Summary
- Nitrogen is a colourless, odourless, and tasteless element that is plentiful in nature.
- Because of the strong triple bond that holds the \({\rm{N}}\) atoms together, \({{\rm{N}}_2}\) is chemically inert. Dinitrogen is a form of nitrogen.
- Chemical exchanges or thermal diffusion can separate the two naturally occurring isotopes of nitrogen, nitrogen-\(14\) and nitrogen-\(15\). Nitrogen has isotopes with mass numbers of \(12, 13, 16\), and \(17\), but they are radioactive.
- Commercial nitrogen can be obtained through liquefaction and fractional distillation of air.
- Nitrogen is a non-toxic gas that is only sparingly soluble in water and condenses to create a colourless liquid. This, on solidification, results in the formation of snow like mass.
- At normal temperatures, \({{\rm{N}}_2}\) is practically non-reactive. It doesn’t burn and doesn’t enable combustion. It does, however, interact with some metals and non-metals at high temperatures to generate nitrides, which are ionic and covalent compounds.
- The Haber process, which produces ammonia, is the most common commercial application for elemental nitrogen gas.
Frequently Asked Questions (FAQs)
Q.1. Discuss the structure of nitrogen molecules.
Ans. Because of the strong triple bond that holds the \({\rm{N}}\) atoms together, \({{\rm{N}}_2}\) is chemically inert. Dinitrogen is a form of nitrogen. \({{\rm{N}}_2}\) is a gas (bp \({\rm{77}}{\rm{.3}}\,{\rm{K}}\)). \({{\rm{N}}_2}\) makes up \({\rm{78\% }}\) of the atmosphere. \({{\rm{N}}_{\rm{2}}}{\rm{‘s}}\) inert behaviour is attributed to the \({\rm{N}} \equiv {\rm{N}}\) triple bond. Because of its tiny size, it forms strong p-p type pi bonds. \({{\rm{N}}_{\rm{2}}}\) has a small internuclear distance \(\left( {{\rm{1}}{\rm{.094}}\,\mathop {\rm{A}}\limits^{\rm{o}} } \right)\) and the ability to create up to four bonds
Q.2. How nitrogen is obtained from the fractional distillation of air?
Ans. By applying a high pressure of \(100\) to \(200\) atmospheres, we may convert air to liquid air. Following that, we feed the compressed air through a fine jet, where it expands. This technique is repeated multiple times, resulting in the production of liquid air. Fractional distillation is applied to the resulting liquid. Dinitrogen has a lower boiling point than liquid oxygen; therefore, it separates, leaving liquid oxygen behind.
Q.3. What are the physical properties of dinitrogen?
Ans. In nature, nitrogen is colourless, odourless, and diamagnetic. Nitrogen is a non-toxic gas that is only sparingly soluble in water and condenses to create a colourless liquid. This, on solidification, results in the formation of snow like mass.
Q.4. What is the Haber Process?
Ans. At about \({\rm{773}}\,{\rm{K}}\), dinitrogen reacts with hydrogen gas to form ammonia gas. This process is known as the Haber Process.