- Written By
Ankita Sahay
- Last Modified 13-04-2025
Electrochemical Principles of Metallurgy: Processes, Types & Examples
Electrochemical Principles of Metallurgy: Electrochemistry is a branch of chemistry that is concerned with the relation between electric current and chemical reactions. The processes involved in these phenomena are known as Electrochemical processes. In this process, chemical energy helps the movement of electrons that generates electricity. The equipment that brings about such changes where chemical changes are brought about with the help of electricity is known as an ‘Electrochemical cell’.
Electrochemistry is widely used in metallurgy. A branch of science that deals with the properties of metals and their production and purification is defined as Metallurgy. There are many metals that are purified by electrochemical processes. We can say that among all other metallurgical processes, electrochemical metallurgical processes produce the purest quality of metals. Some of them are the production of Aluminium by Hall-Hroult electrolysis, refining of copper by electrolysis, electrolytic recovery of recycled silver that produces silver crystals. This method is also used in electroplating. The electrochemical principle of metallurgy is carried on by the reduction of metal ions to their respective metals in their solution or molten states.
What are Electrochemical Principles?
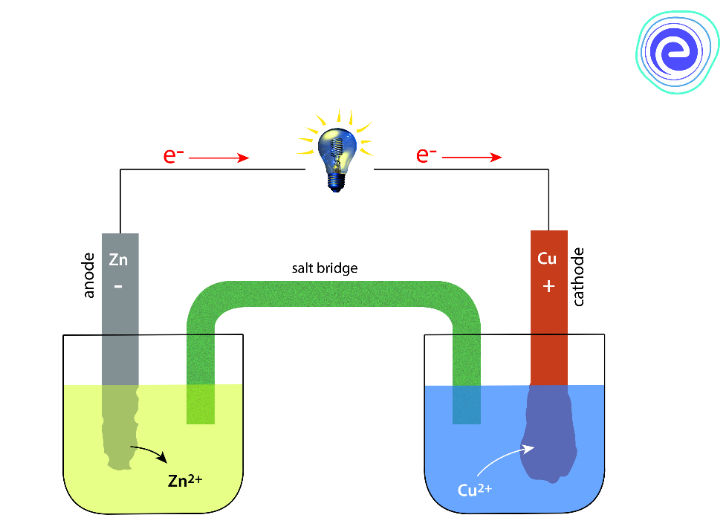
An electrochemical cell works on the principle of redox reaction. It is an apparatus that produces electric current from the energy released by chemical change accompanied by a spontaneous redox reaction. An electrochemical cell contains two half-cells, and each of them consists of an electrode and an electrolyte that can be the same or different in the two half cells. The basic components of an electrochemical cell are:
Learn About Electrochemical Cells Here
1. Electrodes – The solid metallic electrical conductor in an electrochemical cell are known as electrodes. They are of two types:
i. Anode – The cell compartment where oxidation occurs.
ii. Cathode: The cell compartment where reduction occurs.
2. Electrolyte: It is an electrically conducting solution when dissolved in polar solvents, such as water that produces ions.
3. Salt Bridge connects the oxidation half and reduction half of an electrochemical cell and completes the entire electrochemical circuit. It is filled with saturated salt solutions like \({\text{KCl}}{\text{.}}\)
The science or technology of extracting metals from their ores and purifying them for useful purposes to mankind is known as metallurgy. In metallurgy, the physical and chemical behaviour of metallic elements, their inter-metallic compounds, and their mixtures are studied. Metallurgy comprises of \(3\) steps:
I. Concentration of Ore
II. Extraction of metal from its ore
III. Purification of extracted metal
The following are the electrochemical principles of metallurgy:
1. The most important step in this type of metallurgy is the reduction of metal ions to their respective metals in their solution or molten states.
2. Electrolytic reduction is carried out using reducing elements. For example, metal ion and reducing element gives metal and reduced ion as shown in the chemical equation:
\({{\text{M}}^{{\text{n}} + }} + {\text{A}} \to {\text{M}} + {{\text{A}}^{{\text{n + }}}}\)
3. These electrochemical principles are explained on the basis of the equation:
\(\Delta {{\text{G}}^ \circ } = \,- {\text{n}}{{\text{E}}^ \circ }{\text{F}}\)
Where \({\text{n = }}\) Number of electrons gained
\({{\text{E}}^ \circ }{\text{ = }}\) Standard Electrode Potential of Redox reaction.
4. The value of \({{\text{E}}^ \circ }\) for a metal decides its reactivity. Metals that are more reactive have high \({{\text{E}}^ \circ },\) whereas less reactive metals have low \({{\text{E}}^ \circ }.\)
5. Sometimes it is difficult to reduce metals with more negative \({{\text{E}}^ \circ }\) values.
6. If there is the positive difference between the \({{\text{E}}^ \circ }\) values of two metals, then the value of \(\Delta {{\text{G}}^ \circ }\) will be negative.
7. Hence, the less reactive metal will come out of the solution, and the more reactive metal will go inside the solution. For example, in the presence of iron, \({\text{Cu}}\left( {{\text{II}}} \right)\) is reduced to \({\text{Cu}}{\text{.}}\)
\({\text{C}}{{\text{u}}^{{\text{n}} + }} + {\text{Fe}} \to {\text{Cu}} + {\text{F}}{{\text{e}}^{{\text{n}} + }}\)
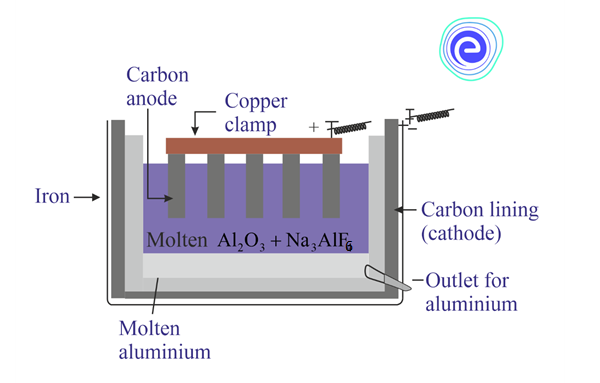
Electrometallurgy is the process of extraction of pure metals by using electricity. Pure Aluminium is extracted by Hall Heroult process discussed below:
1. In this process, alumina \(\left( {{\text{A}}{{\text{l}}_2}{{\text{O}}_3}} \right)\) is dissolved in molten cryolite \(\left( {{\text{N}}{{\text{a}}_3}{\text{Al}}{{\text{F}}_6}} \right).\) Cryolite lowers its melting point for easier electrolysis and better conductivity of the solution.
2. Aluminium fluoride \(\left( {{\text{Al}}{{\text{F}}_3}} \right)\) is added to the mixture to reduce the melting point further.
3. A molten mixture of alumina, cryolite, and aluminium fluoride is electrolysed by passing a low voltage direct current through it.
4. This leads to the deposition of liquid aluminium metal at the cathode while the oxygen from the alumina combines with carbon present in the anode to produce carbon dioxide.
One of the important techniques within the field of metallurgy of obtaining of metals from their ores by using aqueous solutions for the recovery of metals from ores, their concentration, and recycling or residual materials is known as Hydrometallurgy. Typically, Hydrometallurgy is divided into three general areas:
- Leaching – In this process, the ore is treated with chemicals to convert the valuable metals present in the ores into soluble salts while the impurity remains insoluble. These insoluble impurities can then be washed out later and processed to give the pure metal.
- Solution concentration and purification by solvent extraction or ion exchange.
- Metal or metal compound recovery by electrolysis or precipitation.
Electrochemical Principles of Corrosion
Corrosion is an electrochemical process in which metals and alloys get transformed into oxides, hydroxides, and aqueous salts. In the corrosion process, two reactions occur, i.e., oxidation and reduction.
Rusting of iron metal is a special example of corrosion that occurs in the presence of air and water to form a reddish-brown surface. Iron is oxidised in the presence of air and water. The chemical formula of rust is \({\text{Fe}}_2{{\text{O}}_3}.{\text{x}}{{\text{H}}_2}{\text{O}}\) (where \({\text{x}}\) is variable).
The electrochemical cell reaction involved in this process is discussed below:
Iron loses electrons to form \({\text{F}}{{\text{e}}^{2 + }}\) at the anode.
At anode
\({\text{Fe}}\left( {\text{s}} \right) \to {\text{F}}{{\text{e}}^{2 + }}\left( {{\text{aq}}} \right) + 2{{\text{e}}^ – }\)
\({{\text{H}}_2}{\text{O}} \to {{\text{H}}^ + } + {\text{O}}{{\text{H}}^ – }\)
\({\text{C}}{{\text{O}}_2} + {{\text{H}}_2}{\text{O}} \to {{\text{H}}^ + } + {\text{HCO}}_3^ – \)
In the cathode, \({{\text{H}}^ + }\) ions obtained from \({{\text{H}}_2}{\text{O}}\) or acid present in the environment take up the electrons released by the anode, leading to the formation of \({{\text{H}}_2}{\text{O}}{\text{.}}\)
At cathode
\({{\text{H}}^ + } + {{\text{e}}^ – } \to {\text{H}}\)
\(4{\text{H}} + {{\text{O}}_2} \to 2{{\text{H}}_2}{\text{O}}\)
\(4{{\text{H}}^ + } + {{\text{O}}_2} + 4{{\text{e}}^ – } \to 2{{\text{H}}_2}{\text{O}}\)
The ferrous ions are further oxidised to ferric ions to form a compound with \({{\text{H}}_2}{\text{O}}{\text{.}}\)
Overall Cell Reaction in Rusting
\(2{\text{Fe}}\left( {\text{s}} \right) + 4{{\text{H}}^ + } + {{\text{O}}_2} \to 2{\text{F}}{{\text{e}}^{2 + }}\left( {{\text{aq}}} \right) + 2{{\text{H}}_2}{\text{O}}\)
\(4{\text{Fe}} + 3{{\text{O}}_2} \to 2{\text{F}}{{\text{e}}_2}{{\text{O}}_3}\)
\({\text{F}}{{\text{e}}_2}{{\text{O}}_3} + {\text{x}}{{\text{H}}_2}{\text{O}} \to {\text{F}}{{\text{e}}_2}{{\text{O}}_3} \cdot {\text{x}}{{\text{H}}_2}{\text{O}}\left( {{\text{rust}}} \right)\)
Besides reductions, some extractions are based on oxidation particularly for non-metals. A very common example of extraction based on oxidation is the extraction of chlorine from brine (chlorine is abundant in sea water as common salt).
Oxidation and Reduction
\(2{\text{C}}{{\text{l}}^ – }\left( {{\text{aq}}} \right) + 2{{\text{H}}_2}{\text{O}}\left( {\text{l}} \right) \to 2{\text{O}}{{\text{H}}^ – }\left( {{\text{aq}}} \right) + {{\text{H}}_2}\left( {\text{g}} \right) + {\text{C}}{{\text{l}}_2}\left( {\text{g}} \right)\)
The \(\Delta {{\text{G}}^ \ominus }\) for this reaction is \( + 422\,{\text{kJ}}\). When it is converted to \({{\text{E}}^ \ominus }\) (using \(\Delta {{\text{G}}^ \ominus } = – {\text{n}}{{\text{E}}^ \ominus }{\text{F}}\)), we get \({{\text{E}}^ \ominus } = – 2.2\;{\text{V}}\). Naturally, it will require an external e.m.f. that is greater than \(2.2\;{\text{V}}\). But the electrolysis requires an excess potential to overcome some other hindering reactions. Thus, \({\text{C}}{{\text{l}}_2}\) is obtained by electrolysis giving out \({{\text{H}}_2}\) and aqueous \({\text{NaOH}}\) as byproducts. Electrolysis of molten \({\text{NaCl}}\) is also carried out. But in that case, \({\text{Na}}\) metal is produced and not \({\text{NaOH}}\).
As studied earlier, extraction of gold and silver involves leaching the metal with \({\text{C}}{{\text{N}}^ – }\). This is also an oxidation reaction \(\left( {{\text{Ag}} \to {\text{A}}{{\text{g}}^ + }\,{\text{or}}\,{\text{Au}} \to {\text{A}}{{\text{u}}^ + }} \right)\). The metal is later recovered by displacement method.
\(4{\text{Au}}\left( {\text{s}} \right) + 8{\text{C}}{{\text{N}}^ – }\left( {{\text{aq}}} \right) + 2{{\text{H}}_2}{\text{O}}\left( {{\text{aq}}} \right) + {{\text{O}}_2}\left( {\text{g}} \right) \to 4{\left[ {{\text{Au}}{{\left( {{\text{CN}}} \right)}_2}} \right]^ – }\left( {{\text{aq}}} \right) + 4{\text{O}}{{\text{H}}^ – }\left( {{\text{aq}}} \right)\)
\(2{\left[ {{\text{Au}}{{\left( {{\text{CN}}} \right)}_2}} \right]^ – }\left( {{\text{aq}}} \right) + {\text{Zn}}\left( {\text{s}} \right) \to 2{\text{Au}}\left( {\text{s}} \right) + {\left[ {{\text{Zn}}{{\left( {{\text{CN}}} \right)}_4}} \right]^{2 – }}\left( {{\text{aq}}} \right)\)
In this reaction zinc acts as a reducing agent.
In a nutshell, we can say that the branch of science that deals with the properties of metals and their production and purification are defined as Metallurgy, and Electrometallurgy is the process of extraction of pure metals by using electricity. This process is carried out with the help of an electrochemical cell. An electrochemical cell works on the principle of redox reaction. It is an apparatus that produces electric current from the energy released by chemical change accompanied by a spontaneous redox reaction. One of the most important steps in electrochemical metallurgy is the reduction of metal ions to their respective metals in their solution or molten states. Pure Aluminium is extracted by the Hall Heroult process, which is an electrochemical metallurgical process. Hydrometallurgy is another type of metallurgy process. Thus, among all other metallurgical processes, electrochemical metallurgical processes produce the purest quality of metals.
Q.1. What are the principles of metallurgy?
Ans: In metallurgy, the physical and chemical behaviour of metallic elements, their inter-metallic compounds, and their mixtures are studied. Metallurgy comprises 3 basic principles: Concentration of Ore, Extraction of metal from its ore, and Purification of extracted metal.
Q.2. What is the electrometallurgical method?
Ans: The process of extracting pure metals from their ores by using electricity is known as the electrometallurgical method. The most important step in this type of metallurgy is the reduction of metal ions to their respective metals in their solution or molten states.
Q.3. What are the thermodynamic principles of metallurgy?
Ans: The main thermodynamic principle of metallurgy is Gibbs Energy. In thermodynamics, whether a process will take place spontaneously or not will be determined by Gibbs Energy. If \(\Delta {\text{G}}\) is positive, the reaction is considered to be non-spontaneous, and if \(\Delta {\text{G}}\) is negative, the reaction is considered to be spontaneous.
Q.4. What is the metallurgy process?
Ans: The science or technology of extracting metals from their ores and purifying them for valuable purposes to mankind is known as metallurgy. In metallurgy, the physical and chemical behaviour of metallic elements, their inter-metallic compounds, and their mixtures are studied. Among all other metallurgical processes, electrochemical metallurgical processes produce the purest quality of metals. Some of them include the production of Aluminium by Hall-Hroult electrolysis, refining of copper by electrolysis, electrolytic recovery of recycled silver that produces silver crystals. This method is also used in electroplating.
Q.5. What is metallurgy used for?
Ans: Metallurgy is defined as a process that involves theextraction of metals in their purest form. The compounds of metals that are mined from underground are mixed with soil, limestone, sand, and rocks; these are known as minerals. Metals are commercially extracted from minerals at minimum cost and effort. The entire metallurgical processes include the application of the processes used in the separation and concentration of raw materials. These techniques include different chemical processing to convert minerals from inorganic compounds to metals and other materials that are useful to humans.
Learn About Electrolytic Cell Here
We hope this article on Electrochemical Principles of Metallurgy has helped you. If you have any queries, drop a comment below, and we will get back to you.