- Written By
Sushmita Rout
- Last Modified 15-02-2025
Elevation in Boiling Point of the Solvent: Definition, Formula, Solved Examples
Elevation in Boiling Point of the Solvent: Salt is an interesting ingredient available in our kitchens. It not only adds flavour to our food but also fastens the cooking process. But how does salt fasten the cooking process? It elevates the water’s boiling point. The more salt we add, the higher is the boiling point. Water no longer boils at \(100\) degrees Celsius but at a temperature higher than this. Let’s learn everything about the elevation in the boiling point of the solvent.
What is the Boiling Point?
The temperature at which a liquid boils and changes to its gaseous or vapour form at normal atmospheric pressure is known as the boiling point. It is the temperature at which both the liquid and gaseous phases of a substance coexist in equilibrium. At this temperature, the vapour pressure of the liquid is equal to its external pressure.
What is the Elevation in Boiling Point?
When a non-volatile solute, such as salt, is added to a volatile liquid solvent, the resulting solution has a lower vapour pressure than that of the pure solvent. Hence, the temperature required by the liquid solvent to reach its gaseous state increases. This results in the elevation of the boiling point of the solution compared to the solvent.
The elevation of the boiling point of the solution is regarded as one of the colligative properties of a solution as it depends on the number of solute particles present in the solution.
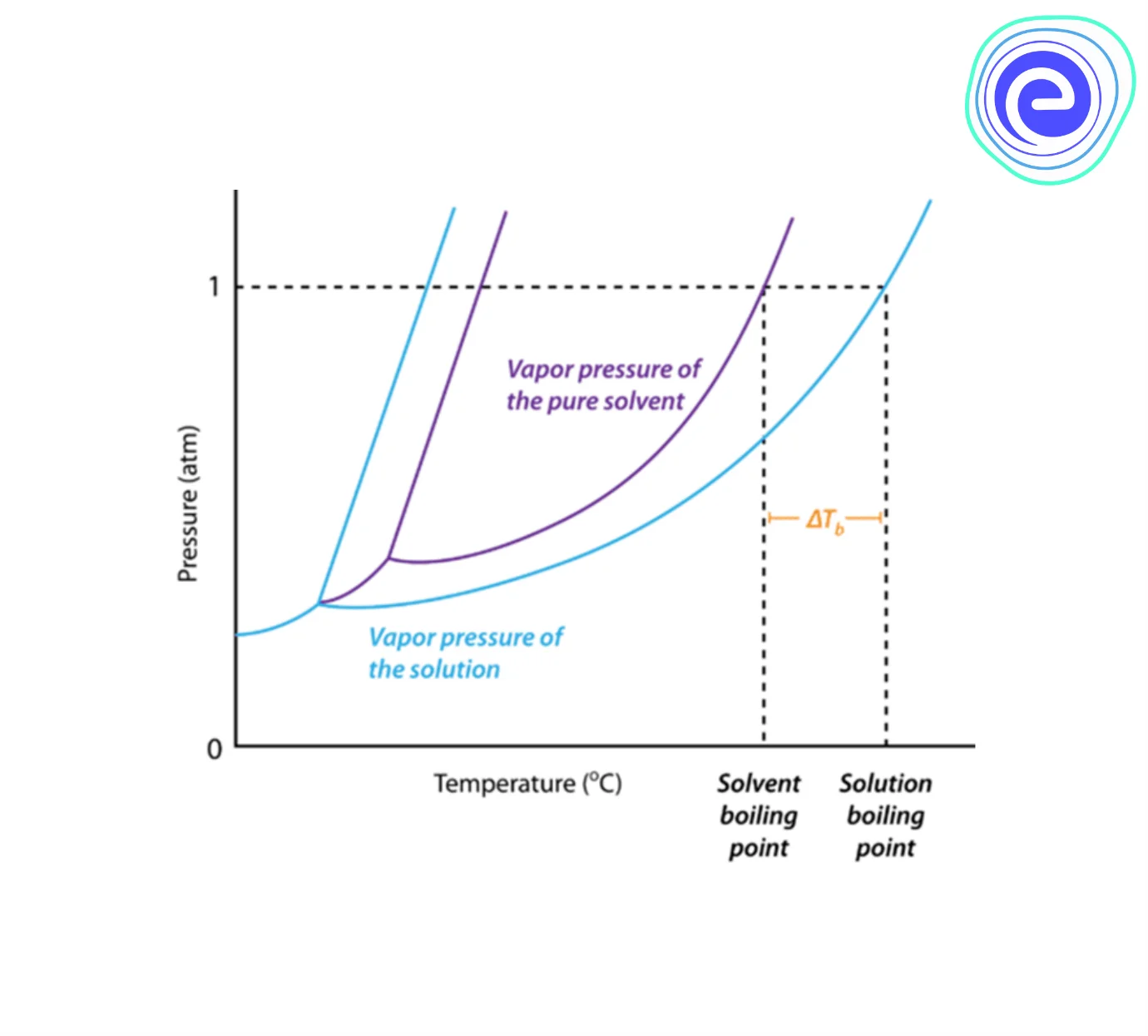
The elevation of the boiling point of the solution can be defined as the difference in temperature between the boiling point of the solution to that of the pure solvent. On the graph below, the boiling point elevation is represented by \(\Delta {{\rm{T}}_{\rm{b}}}\).
The vapour pressure of a solution (blue) is lower than that of a pure solvent (purple). As a result, the boiling point of a solvent increases when any non-volatile solute is dissolved into it.
Why does an Elevation in the Boiling Point of the Solvent Occur on Adding a Non-Volatile Solute?
On adding a non-volatile solute to a volatile solvent, an elevation in the solvent’s boiling point occurs because it depends on the number of particles formed in the solution as it is a colligative property. When sodium chloride is added to water, it dissociates into sodium and chloride ions. These ions alter the intermolecular forces between water molecules. Consequently, there is an elevation in the boiling point of the solvent.
The elevation in the solvent’s boiling point is the temperature difference between the boiling point of the solution and that of the pure solvent.
\(\Delta {{\rm{T}}_{\rm{b}}} = {{\rm{T}}_{\rm{b}}} – {\rm{T}}_{\rm{b}}^0\)
Where, \({\rm{T}}_{\rm{b}}^0 = \) Boiling point of pure solvent
\({{\rm{T}}_{\rm{b}}} = \) Boiling point of the solution (non-volatile solute + solvent)
For a dilute solution, the Elevation in the boiling point of the solvent \(\Delta {{\rm{T}}_{\rm{b}}}\) varies directly with the molality of the solution. Mathematically,
\(\Delta {{\rm{T}}_{\rm{b}}} \propto {\rm{m}}\)
\(\Delta {{\text{T}}_{\text{b}}} = {{\text{K}}_{\text{b}}}{\text{m}}……{\text{Eqn}}\left( 1 \right)\)
Here, \({{\rm{K}}_{\rm{b}}}\) is the proportionality constant, also known as boiling point elevation constant or molal elevation constant (Ebullioscopic Constant). It depends upon the nature of the solvent. The unit of \({{\rm{K}}_{\rm{b}}}\) is \({\rm{K}}\,{\rm{kg}}\,{\rm{mo}}{{\rm{l}}^{ – 1}}\).
Let \({{\rm{w}}_2}\) gram of the solute with \({{\rm{M}}_2}\) molar mass is present in \({{\rm{w}}_1}\) gram of the solvent. If the resulting solution produces an elevation in the boiling point \(\Delta {{\rm{T}}_{\rm{b}}}\) of the solvent, then molality of the solute is given by-
Molality of the solute \(({\rm{m}}) = \frac{{{{\rm{w}}_2}/{{\rm{M}}_2}}}{{{{\rm{w}}_1}/1000}}\)
Substituting the above value in Eqn\((1)\), we get-
\(\Delta {{\rm{T}}_{\rm{b}}} = \frac{{{{\rm{K}}_{\rm{b}}} \times {{\rm{w}}_2}/{{\rm{M}}_2}}}{{{{\rm{w}}_1}/1000}}\)
\(\Delta {{\rm{T}}_{\rm{b}}} = \frac{{{{\rm{K}}_{\rm{b}}} \times {{\rm{w}}_2} \times 1000}}{{{{\rm{w}}_1} \times {{\rm{M}}_2}}}\)
\({{\rm{M}}_{\rm{2}}}{\rm{ = }}\frac{{{{\rm{K}}_{\rm{b}}}{\rm{ \times }}{{\rm{w}}_{\rm{2}}}{\rm{ \times 1000}}}}{{{{\rm{w}}_{\rm{1}}}{\rm{ \times \Delta }}{{\rm{T}}_{\rm{b}}}}}\)
The value of \({{\rm{K}}_{\rm{b}}}\) can be determined from the following relations-
\({{\rm{K}}_{\rm{b}}}{\rm{ = }}\frac{{{\rm{R \times }}{{\rm{M}}_{\rm{1}}}{\rm{ \times T}}_{\rm{b}}^{\rm{2}}}}{{{\rm{1000 \times }}{{\rm{\Delta }}_{{\rm{fus}}}}{\rm{H}}}}\)
Where \({{\rm{R = }}}\) gas constant
\({{{\rm{M}}_{\rm{1}}}{\rm{ = }}}\) molar mass of the solvent
\({{{\rm{\Delta }}_{{\rm{fus}}}}{\rm{H = }}}\) enthalpies for the fusion of the solvent.
Consequences of Boiling Point Elevation
Antifreeze
Ethylene glycol or antifreeze is used to prevent thezing of water in the vehicle’s radiator. It does so by elevating the boiling point of the fluid.
Cooking
The boiling point of water will increase if we add salt before or while heating. The water will boil at a temperature above 100 degrees Celsius and enhance the cooking process. The amount of this increase is, however, quite negligible at low concentrations of salt.
Measurement of Molar Mass
Boiling point elevation depends on the identity and nature of the solvent molecules and the concentration of solute particles. However, it is independent of the identity and nature of the solute particles. Consequently, just like depression inzing point, Elevation in boiling point can be used to determine the molar mass of a solute.
Sugar Refining
During the process of sugarcane refining, the cane syrup is boiled, and the temperature at which it boils depends on the concentration of sugar. The solution’s saturation level is monitored through the boiling point elevation, which is an important consideration for crystallization.
Solved Examples on Elevation in Boiling Point of the Solvent
Q.1. Seawater has \(3.5\%\) of dissolved solids, almost all of which is \({\rm{NaCl}}\). Determine the normal boiling point of seawater.
Ans: \(3.5\%\) means \(3.5\) grams of solids are dissolved in \(100\) grams of the total solution. This means \(3.5\) grams of solids are dissolved in \(96.5\) grams of water.
Calculate the moles of \({\rm{NaCl}}\):
Moles of \({\rm{NaCl}} = \frac{{{\rm{ Mass}}\,{\rm{of \,NaCl}}}}{{{\rm{ Molar}}\,{\rm{mass}}\,{\rm{of}}\,{\rm{NaCl}}}} = \frac{{3.5}}{{58.5}} = 0.0598\;\,{\rm{mol}}/{\rm{Kg}}\)
Calculate the molality of \({\rm{NaCl}}\):
Molality of \({\rm{NaCl}}({\rm{m}}) = \frac{{{\rm{ Moles}}\,{\rm{of}}\,{\rm{NaCl}}}}{{{\rm{ Amount}}\,{\rm{of}}\,{\rm{the}}\,{\rm{solvent}}\,{\rm{in}}\,{\rm{Kg}}}} = \frac{{0.0598\;{\rm{mol}}}}{{0.0965\;{\rm{kg}}}} = 0.612\;{\rm{mol}}/{\rm{Kg}}\)
Calculating the Van’t Hoff factor (i) for \({\rm{NaCl}}\):
\({\text{NaCl}} \rightleftharpoons {\text{N}}{{\text{a}}^ + } + {\text{C}}{{\text{l}}^ – }\)
\({\rm{i}} = 2\)
Using boiling point elevation constant:
\(\Delta {\rm{T}} = {\rm{i}}{{\rm{K}}_{\rm{b}}}{\rm{m}} = (2)\left( {{{0.52}^{\rm{o}}}{\rm{C\,Kg}}/{\rm{mol}}} \right)(0.612\;{\rm{mol}}/{\rm{Kg}}) = {0.64^{\rm{o}}}{\rm{C}}\)
So, the water boils at \({100.64^{\rm{o}}}{\rm{C}}\).
Summary of Elevation in Boiling Point of the Solvent
We now know the science behind adding salt to boiling eggs. On adding salt to the water, due to vapour pressure lowering, the boiling point of the solution increases, whereas itszing point decreases. All these are consequences of the addition of a non-volatile solute to a volatile solvent. This article explains the concept of boiling point, the reason behind the elevation in the boiling point of the solvent and its significant consequences.
Frequently Asked Questions on Elevation in Boiling Point of the Solvent
Q.1. What is boiling point elevation?
Ans: The boiling point elevation refers to the rise in the solvent’s boiling point upon the addition of a solute. When a non-volatile solute is added to a solvent, then the resulting solution has a higher boiling point.
Q.2. Why does boiling point elevation occur?
Ans: When a non-volatile solute is added to a solvent, the vapour pressure of the resulting solution becomes less than the vapour pressure of the pure solvent. The boiling point of a solution will then be greater than the boiling point of the pure solvent. This is because a lower vapour pressure will need a higher temperature to become equal to the external pressure (i.e., the boiling point).
Q.3. How does solute affect boiling point?
Ans: A solute raises the boiling point of the resulting solution compared to the pure solvent. The amount by which the boiling point increases depends on the concentration of particles but not on the identity of the solute.
Q.4. Which solution has the highest boiling point?
Ans: The boiling point of a solution depends on ‘i’ or the van’t Hoff factor. ‘i’ represents the number of particles produced after ionization or dissolution. So, the more the value of ‘i’, the more the number of particles and the higher the boiling point will be.
Q.5. What is the boiling point elevation constant?
Ans: The boiling point elevation constant \({{\rm{K}}_{\rm{b}}}\) is a constant which is equal to the change in the boiling point for a \(1\)-molal solution of a non-volatile molecular solute. For water, the value of \({{\rm{K}}_{\rm{b}}}\) is \(0.512\,{\rm{K}}\,{\rm{Kg}}/{\rm{mol}}\). So, the boiling temperature of a \(1\)-molal aqueous solution of any non-volatile molecular solute is \(100.512\,{\rm{K}}\).
Know About The Liquid State Here
We hope this article on the Elevation in Boiling Point of the Solvent has helped you. If you have any queries, drop a comment below and we will get back to you at the earliest.