- Written By
Akanksha P John
- Last Modified 18-01-2025
Group 14 Elements: The Carbon Family- Introduction, Properties, Uses
Group 14 Elements: Do you know about the element carbon? If yes, then you must be well aware that carbon plays a very important role in our life, whether it is in plant or animal life. It plays a major role in respiration as well as in the environment and many other important things.
In this article, let’s study everything about carbon and its family that is our group \(14\) elements- its characteristics, electronic configuration, and some uses.
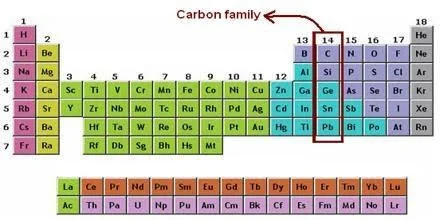
What is Carbon Family?
The carbon family belongs to the periodic table’s group \(14.\) Carbon, silicon, germanium, tin, and lead are the five elements that make up the carbon family. Flerovium, element \(114,\) is likely to behave in some ways like a member of the family as well.
In other words, the group consists of carbon and the elements directly below it in the periodic table. The carbon family belongs to the p-block of elements and is almost in the centre of the periodic table, with nonmetals to its right and metals to its left.
This family was known as the tetrels or tetragens because the elements belonged to group IV or because atoms of these elements had four valence electrons. The crystallogens are another name for this family.
General Characteristics of Group 14 Elements
The important characteristics of group \(14\) elements are as follows:
Electronic Configuration
In these elements, the differentiating electrons enter into ‘np’ subshells. They have four electrons in their outermost shell and possess an electronic configuration of the type \({\rm{n}}{{\rm{s}}^2}{\rm{n}}{{\rm{p}}^2}.\)
Atomic and Ionic Radii
The atomic radius and ionic radius increase as you move down the periodic table in the carbon family, while electronegativity and ionisation energy decrease. As one moves down the group, the size of the atom increases due to the addition of an additional electron shell.
Elements Density
The densities of group \(14\) elements increase in going from carbon to lead. The increase is slow in going from \({\rm{C}}\) to \({\rm{Si}}\) but becomes rapid on further moving down the group. This is due to the more effective packing of the constituent particles in the higher elements.
Elements
There is one nonmetal (carbon), two metalloids (silicon and germanium), and two metals in the carbon family (tin and lead). In other words, as you move down the group, the elements become more metallic.
Melting and Boiling Point
Elements in Group \(14\) (the carbon family) have much higher melting and boiling points than elements in Group \(13.\) Melting and boiling points in the carbon family tend to decrease as one moves down the group, owing to weaker atomic forces within larger molecules. Lead, for example, has such a low melting point that a flame can easily liquefy it.
Ionisation Enthalpy
The first ionisation enthalpy of group \(14\) elements are higher than those of corresponding elements of group \(13,\) and they tend to decrease on moving down the group.
Electronegativity
The group \(14\) elements have higher values of electronegativity than those of the corresponding elements of group \(13.\) The electronegativity decreases in going from \({\rm{C}}\) to \({\rm{Si}}\) and then assumes an almost constant value for the remaining elements.
Electropositive
The group \(14\) elements are less electropositive and hence less metallic than the elements of group \(13.\) On moving down the group, the metallic character increases from \({\rm{C}}\) to \({\rm{Pb}}{\rm{.}}\)
Allotropy
All the elements of group \(14\) except lead show allotropy and exist in different allotropic forms.
Catenation
The ability of an element to form long chains or ring structures by linking its atoms with one another through covalent bonds is called catenation. On moving down the group, the catenation tendency decreases. The variation of the tendency for catenation in going from carbon to lead follows the order.
\({\rm{C}} \gg {\rm{Si}} > {\rm{Ge}} \approx {\rm{Sn}} \gg {\rm{Pb}}\)
Oxidation States and Nature of Compounds
The common oxidation states shown by the elements of group \(14\) are \(+4\) and \(+2.\) On moving down the group, the stability of the \(+4\) state decreases while that of \(+ 2\) state increases. Carbon and silicon form covalent compounds, while the other elements form covalent as well as ionic compounds.
Chemical Properties of Group 14 Elements
Some of the important chemical properties of Group \(14\) elements are explained below:
- Hydrides: The elements of group \(14\) form covalent of the type \({\rm{M}}{{\rm{H}}_4}.\) The number of hydrides, their thermal stability and their ease of formation decrease markedly on going from carbon to lead.
- Halides: The elements of group \(14\) form two types of halides- the tetrahalides of the type \({\rm{M}}{{\rm{X}}_4}\) and dihalides of the type \({\rm{M}}{{\rm{X}}_2}.\)
- Oxides: The elements of group \(14\) form mainly two types of oxides- monoxides of the type \({\rm{MO}}\) and dioxides of the type \({\rm{M}}{{\rm{O}}_2}.\)
\({\rm{CO}}\) is a neutral monoxide, \({\rm{GeO,}}\,{\rm{SnO}}\) and \({\rm{PbO}}\) are amphoteric. The acidic character of the dioxides decreases as one moves down the group. Among the dioxides, \({\rm{C}}{{\rm{O}}_2}\) is the most acidic, while \({\rm{Pb}}{{\rm{O}}_2}\) is the most basic.
Anomalous Behaviour of Carbon
Carbon is the first element of the group, differs from the rest of its group in several properties and thus shows an anomalous behaviour. This behaviour of carbon may be due to the following factors, which are as follows:
- Its small atomic size.
- Its higher electronegativity.
- The absence of vacant d-orbitals in its valence shell.
- Its strong catenation tendency.
The important properties in which carbon differs from the other elements of the group are as follows:
- Carbon is much harder than the other elements of the group. Diamond, an allotrope of carbon, is the hardest substance known.
- The melting and boiling points of carbon are much higher as compared to those of other elements of the group.
Elements of the Carbon Family
Carbon
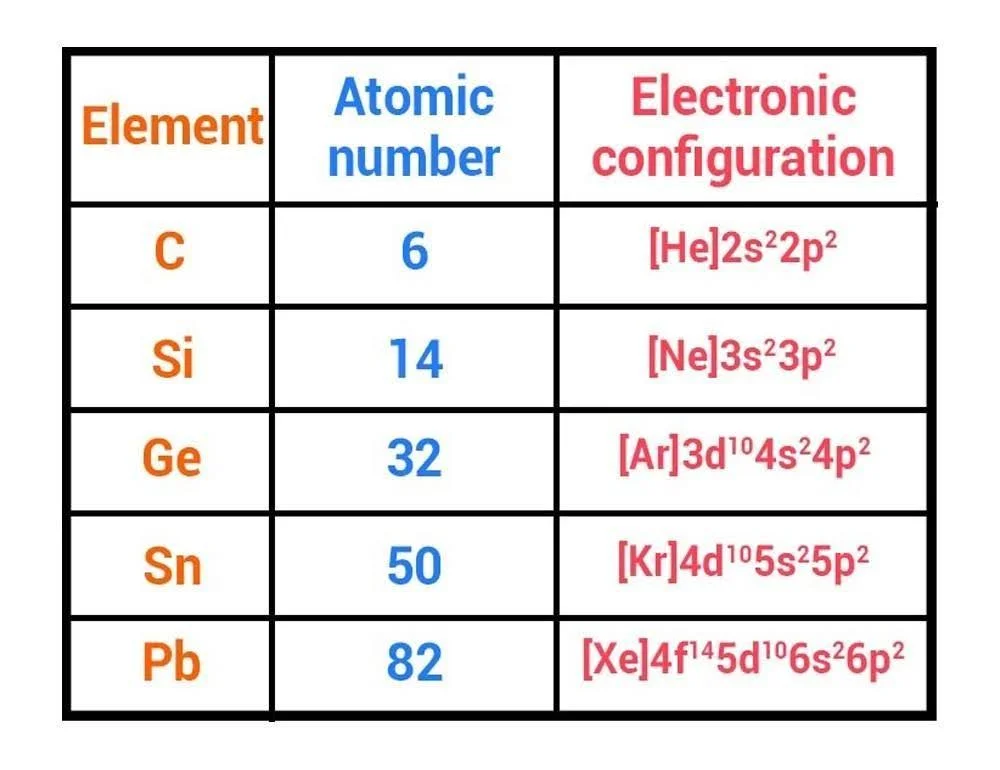
- Atomic number- \(6\)
- Symbol- \({\rm{C}}\)
- Electronic configuration- \({\rm{1}}{{\rm{s}}^2}2{{\rm{s}}^2}2{{\rm{p}}^2}\)
The first element in this \(14\)th group of elements is carbon. It is one of the most abundant elements found on our planet. It can be found in both combined and states. It is commonly found in air, polymers, organic compounds, carbonates, and other materials. It has three isotopes: \({}_6^{12}{\rm{C}},\,{}_6^{13}{\rm{C}}\) and \({}_6^{14}{\rm{C}},\) out of the these \({}_6^{14}{\rm{C}}\) is radioactive.
Uses of Carbon
- In the smelting process of metals, impure carbon in the form of charcoal derived from wood and coke derived from coal is used.
- Graphite is used to make pencils, brushes in electric motors, and furnace linings. Activated charcoal is used in purification and filtration, as well as in respirators.
- Carbon fibre is also used as a very strong, lightweight material. It is now found in tennis rackets, skis, fishing rods, rockets, and aeroplanes.
Silicon
- Atomic number- \(14\)
- Symbol- \({\rm{Si}}\)
- Electronic configuration- \(1{{\rm{s}}^2}2{{\rm{s}}^2}2{{\rm{p}}^6}3{{\rm{s}}^2}3{{\rm{p}}^2}\)
Silicon is the second most common element found in the earth’s crust (after oxygen) and is regarded as the mineral world’s backbone. It is classified as a metalloid rather than a metal or a nonmetal.
Uses of Silicon
- Silicon can be found on sandy beaches and is an important component of concrete and brick.
- Since it is a semiconductor, it is used to make transistors.
- It is most commonly found in computer chips and solar cells.
- It can be used in the production of fire bricks.
- Silicones are used as a component in the majority of waterproofing systems.
- Silicon can be found in a wide range of moulds and moulding compounds.
Germanium
- Atomic number- \(32\)
- Symbol- Ge
- Electronic configuration- \(1{{\rm{s}}^2}2{{\rm{s}}^2}2{{\rm{p}}^6}3{{\rm{s}}^2}3{{\rm{p}}^6}3{{\rm{d}}^{10}}4{{\rm{s}}^2}4{{\rm{p}}^2}\)
Germanium’s physical and chemical properties are similar to those of silicon. It has a grey-white colour and a crystal structure.
Germaniums Uses
- It is a rare element used in the production of semiconductor devices.
- Germanium, when doped with arsenic and other elements, acts as a semiconductor that can be used as a transistor in electronic applications.
Tin (Stannum)
- Atomic number- \(50\)
- Symbol- \({\rm{Sn}}\)
- Electronic configuration- \(1{{\rm{s}}^2}2{{\rm{s}}^2}2{{\rm{p}}^6}3{{\rm{s}}^2}3{{\rm{p}}^6}3{{\rm{d}}^{10}}4{{\rm{s}}^2}4{{\rm{p}}^6}4{{\rm{d}}^{10}}5{{\rm{s}}^2}5{{\rm{p}}^2}\)
Tin is a malleable, soft metal with a low melting point. It is derived primarily from the mineral cassiterite. At normal pressure and temperature, it has two major allotropes.
Tin Uses
- Because of its high corrosion resistance, it can be used in the processes of tin plating, coating, and polishing.
- It is used in steel soldering because it has high magnetic strengths and lower melting points.
- It can be used to make other alloys such as bronze and copper.
Lead (Plumbum)
- Atomic number- \(82\)
- Symbol- \({\rm{Pb}}\)
- Electronic configuration- \(1{{\rm{s}}^2}2{{\rm{s}}^2}2{{\rm{p}}^6}3{{\rm{s}}^2}3{{\rm{p}}^6}3{{\rm{d}}^{10}}4{{\rm{s}}^2}4{{\rm{p}}^6}4{{\rm{d}}^{10}}4{{\rm{f}}^{14}}5{{\rm{s}}^2}5{{\rm{p}}^2}5{{\rm{d}}^{10}}6{{\rm{s}}^2}6{{\rm{p}}^2}\)
Lead, a soft, silvery-white or greyish metal, and similar to tin it is soft, malleable, and has a low melting point. Lead is toxic to human health, especially to children.
Lead Uses
- It was widely used in water and sewage pipes.
- It is used in heavy and industrial machinery, sheets, and other lead-based parts that can be used to dampen noise and vibration.
Flerovium
- Atomic number- \(114\)
- Symbol- \({\rm{Fl}}\)
It is radioactive and has a very short half-life. Flerovium’s long-lasting isotope has an atomic weight of \(289\) and a half-life of \(0.97\) seconds. The half-lives of three other flerovium isotopes are \(0.52, 0.51\) and \(0.16\) seconds, respectively.
Summary of Group 14 Elements
In this article, we studied in detail about a particular group of the p-block that is the carbon family. Now we know that:
- The carbon family has five elements in it.
- The general physical and chemical properties of the group.
- Uses of each element in the carbon group.
- Anomalous behaviour of carbon.
FAQs on Group 14 Elements
Q.1. Why is group 14 called the carbon family?
Ans: Generally, the name of the group is considered by its first element. Group \(14\) is called the carbon family because the first member of this group is carbon.
Q.2. What does group 14 have in common?
Ans: The elements of group \(14\) have four electrons in their outermost shell and possess electronic configuration of the type \({\rm{n}}{{\rm{s}}^2}{\rm{n}}{{\rm{p}}^2}.\)
Q.3. What member of the carbon family has 14 electrons?
Ans: The member of the carbon family that has \(14\) electrons is silicon \(\left( {{\rm{Si}}} \right).\)
Q.4. What are the characteristics of the carbon family?
Ans: The general characteristics of the carbon family are as follows:
(i) Electronic configuration– In these elements, the differentiating electrons enter into ‘np’ subshells. They possess an electronic configuration of \({\rm{n}}{{\rm{s}}^2}{\rm{n}}{{\rm{p}}^2}.\)
(ii) Atomic and ionic radii– The atomic and ionic radii are smaller than those of group \(13,\) and they tend to increase on moving down the group.
(iii) Density– The densities increase on going from \({\rm{C}}\) to \({\rm{Pb}}{\rm{.}}\)
(iv) Melting and boiling points- The melting and boiling point of \({\rm{C}}\) and \({\rm{Si}}\) are much higher than other elements in the group. On moving down the group, the melting and boiling points decrease.
(v) Oxidation state- The common oxidation states shown by this group are \(+4\) and \(+2.\)
Q.5. What are the elements of the carbon family?
Ans: The carbon family belongs to group \(14\) in the periodic table. Carbon, silicon, germanium, tin, and lead are the five elements that make up the carbon family.
Q.6. What is the electronic configuration of the carbon family?
Ans: In the elements of group \(14,\) the differentiating electrons enter into ‘np’ sub-shells. They possess an electronic configuration of \({\rm{n}}{{\rm{s}}^2}{\rm{n}}{{\rm{p}}^2}.\)
Q.7. How does electronegativity vary along with the group 14 elements?
Ans: The electronegativity decreases on going from \({\rm{C}}\) to \({\rm{Si}}\) and then assumes an almost constant value for the remaining elements.
We hope this article on the Group \(14\) elements will be helpful to you in your preparation. If you have any doubts related to the article or in general about the Group \(14\) elements, please reach out to us through the comments section, and we will get back to you as soon as possible.