- Written By
Ankita Sahay
- Last Modified 31-12-2024
Identification of Copper Oxide and Manganese Oxide
Identification of Copper Oxide and Maganese Oxide: A chemical compound that contains at least one oxygen atom along with one another element is known as an oxide. Metal oxide contains an oxygen anion having an oxidation state \(-2\). Among different metal oxides, transition metals have variable oxidation states and hence form different oxides. In this article, we will focus on the oxides of two transition metals: copper and manganese.
Copper exhibits oxidation states of \(+1\) and \(+2\), whereas manganese exhibits variable oxidation states like \(+2,+3,+4\), to \(+7\). Copper oxides exist in their most common forms as copper (I) oxide and copper (II) oxide, which are formed when oxygen combines with copper in different ways. On the other hand, Manganese oxides are found in various oxidation states such as \(\mathrm{MnO}, \mathrm{MnO}_{2}, \mathrm{Mn}_{2} \mathrm{O}_{3}, \mathrm{Mn}_{3} \mathrm{O}_{4}\), etc. There are many physical and chemical properties that can be used to identify the various oxides of copper and manganese.
Copper Oxide
1. Cuprous oxide \(\left(\mathrm{Cu}_{2} \mathrm{O}\right)\) is a copper oxide where copper exists in the liquid form. In \(\mathrm{Cu}_{2} \mathrm{O}\), copper and oxygen are bonded through the covalent bond. Crystals of cuprous oxide are found in cubic shape.
2. The solution of \(\mathrm{Cu}_{2} \mathrm{O}\) gets readily reduced when heated in the presence of hydrogen.
3. In an acidic solution, cuprous oxide gets disproportionated to produce copper and copper (II) ions. The colour of copper (I) oxide \(\left(\mathrm{Cu}_{2} \mathrm{O}\right)\) is red, and the colour of Copper (II) oxide \((\mathrm{CuO})\) is black.
Uses of Copper Oxide
1. Copper oxides are used for building copper-based structures. These types of structures gradually change colour due to oxidation.
2. As we know that copper oxides behave as superconductive at higher temperatures; thus, they are used in producing photoelectric cells in solar panels.
3. Copper oxides are used in agricultural use as fungicides and pesticides.
Manganese Oxide
Apart from manganese as a metal, manganese oxides are a wide range of compounds with a wide variety of uses. The oxidation state of the manganese ranges from \(+2\) to \(+7\), with one oxide bearing both \(+2\) and \(+3\) oxidation states. In total, there are six oxides of manganese.
Given below is the list of various Manganese oxides:
1. Manganese (II) oxide, \(\text {MnO}\)
2. Manganese (III) oxide, \(\mathrm{Mn}_{2} \mathrm{O}_{3}\)
3. Manganese (II, III) oxide, \(\mathrm{Mn}_{3} \mathrm{O}_{4}\)
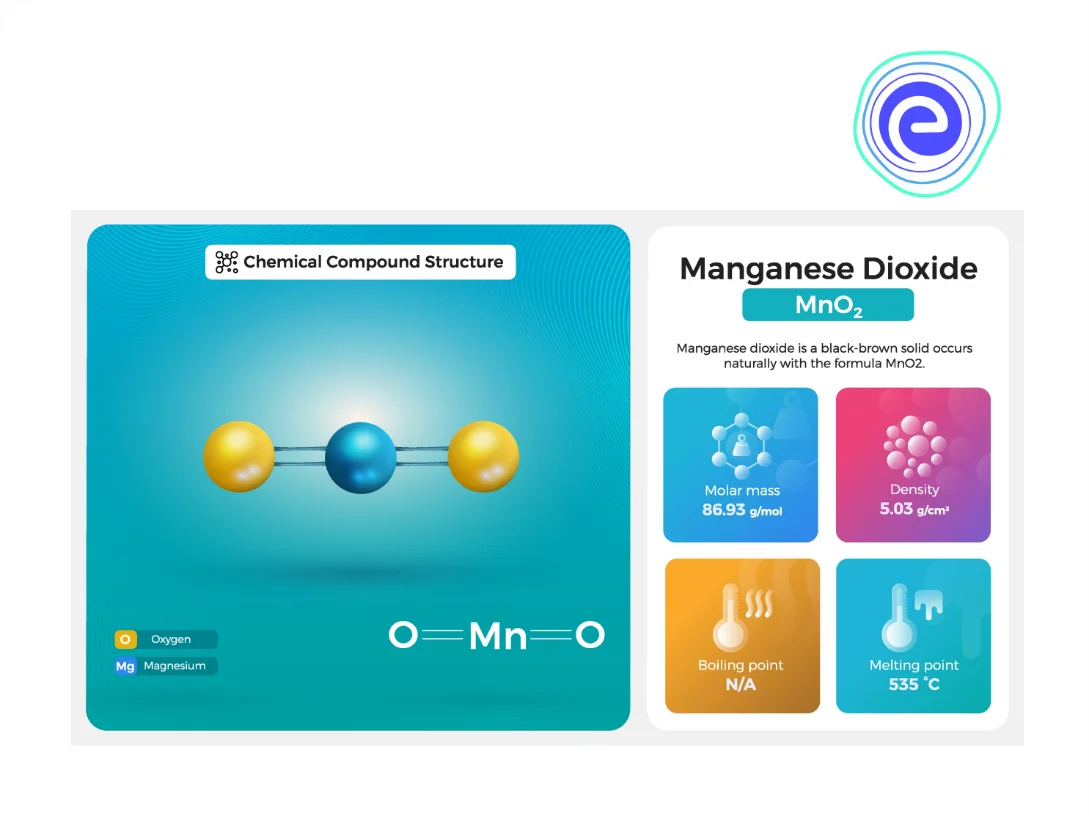
4. Manganese dioxide or Manganese (IV) oxide, \(\mathrm{MnO}_{2}\)
5. Manganese (VI) oxide, \(\mathrm{MnO}_{3}\)
6. Manganese (VII) oxide, \(\mathrm{Mn}_{2} \mathrm{O}_{7}\)
7. Many other manganese oxides exist, such as \(\text {Mn}_{5} \text {O}_{8}, \text {Mn}_{7} \text {O}_{13}\) and \(\text {Mn}_{7} \text {O}_{12}\).
Uses of Manganese Oxide
- Manganese oxides are mostly used in bricks and ceramic products that give rich brown, blue, grey, and black colour to the finished product, along with some added benefits in terms of handling and durability.
- Manganese oxides occur naturally in mines and are used in the production of Manganese metal too.
- Manganese oxide is used in the health industry, chemical industries, oil, glass and animal feed.
Distinguishing Copper Oxide and Manganese Dioxide
We can identify Copper oxide and Manganese dioxide by various chemical tests discussed below:
1. When concentrated hydrochloric acid is added to manganese dioxide, it produces chlorine with greenish-yellow gas, which turns moist starch iodide paper blue-black. The chemical reaction involved in this process is given below:
\(\mathrm{MnO}_{2}+4 \mathrm{HCl} \rightarrow \mathrm{MnCl}_{2}+2 \mathrm{H}_{2} \mathrm{O}+\mathrm{Cl}_{2}\)
On the other hand, when the same procedure is carried out with copper oxide, i.e., when the copper oxide is heated with concentrated hydrochloric acid, it does not form any chlorine.
\(\mathrm{CuO}+\mathrm{HCl} \rightarrow \mathrm{CuCl}_{2}+\mathrm{H}_{2} \mathrm{O}\)
2. In both the above cases, when the solution of \(\mathrm{MnCl}_{2}, \mathrm{H}_{2} \mathrm{O}\), and \(\mathrm{Cl}_{2}\) is filtered, the filtrate obtained is brownish in colour. In contrast, when the solution of \(\mathrm{CuCl}_{2}\) and \(\mathrm{H}_{2} \mathrm{O}\) is filtered, the filtrate obtained is bluish in colour.
3. Another confirmatory test can be done by adding Ammonium hydroxide \(\left(\mathrm{NH}_{4} \mathrm{OH}\right)\) to the solution of the filtrate from Manganese dioxide, no precipitate is formed, while filtrate from copper oxide gives pale blue precipitate on addition of \(\mathrm{NH}_{4} \mathrm{OH}\), and on excess \(\mathrm{NH}_{4} \mathrm{OH}\), the precipitate turns dark blue.
4. Based on the structures of \(\mathrm{MnO}_{2}\) and \(\mathrm{CuO}, \mathrm{MnO}_{2}\) contains \(\mathrm{Mn}^{4+}\) ions and \(\mathrm{O}^{2-}\) ions. At the same time, \(\mathrm{CuO}\) contains \(\mathrm{Cu}^{2+}\) ions and \(\text {O}^{2-}\) ions.
In short, we have learned about different oxides of manganese and copper in this article. As compared to copper oxides, copper (I) oxide and copper (II) oxide, manganese oxides exist in various forms such as manganese (II) oxide ( \(\mathrm{MnO}\) ), manganese (III) oxide \(\left(\mathrm{Mn}_{2} \mathrm{O}_{3}\right)\), manganese (II, III) oxide, \(\left(\mathrm{Mn}_{3} \mathrm{O}_{4}\right)\), manganese dioxide or manganese (IV) oxide \(\left(\mathrm{MnO}_{2}\right)\), manganese \((\mathrm{VI})\) oxide \(\left(\mathrm{MnO}_{3}\right)\), manganese \((\mathrm{VII})\) oxide \(\left(\mathrm{Mn}_{2} \mathrm{O}_{7}\right)\) and many others like \(\mathrm{Mn}_{5} \mathrm{O}_{8}\), \(\mathrm{Mn}_{7} \mathrm{O}_{13}\) and \(\mathrm{Mn}_{7} \mathrm{O}_{12}\). The colour of copper (I) oxide \(\left(\mathrm{Cu}_{2} \mathrm{O}\right)\) is reddish, and the colour of copper (II) oxide (\(\mathrm{CuO}\)) is blackish. Copper oxides are used in different fields, like in building copper-based structures and statues. They are used in producing photoelectric cells in solar panels because copper oxides behave as superconductive at higher temperatures, copper oxides are used in agricultural use as fungicides and pesticides.
Similarly, manganese oxides are mostly used in bricks and ceramic products to give rich brown, blue, grey, and black colour to the finished products; they are used in the production of manganese metal too from minerals, manganese oxides have other uses in the health industry, chemical industries, oil, glass and in animal feed. There are a few distinguishing tests used to identify manganese dioxide and copper oxide. When concentrated hydrochloric acid is added to manganese dioxide, it produces chlorine with greenish-yellow gas, which turns moist starch iodide paper blue-black, and copper oxide is heated with concentrated hydrochloric acid, it does not form any chlorine. On adding ammonium hydroxide \(\left(\mathrm{NH}_{4} \mathrm{OH}\right)\) to the solution of the filtrate from manganese dioxide, no precipitate is formed, while filtrate from copper oxide gives pale blue precipitate on addition of \(\mathrm{NH}_{4} \mathrm{OH}\), and on excess \(\mathrm{NH}_{4} \mathrm{OH}\), the precipitate turns dark blue colour. This helps us to distinguish between \(\mathrm{MnO}_{2}\) and \(\mathrm{CuO}\).
FAQs on Copper Oxide and Manganese Oxide
Q.1. How can you distinguish between manganese dioxide and copper oxide?
Ans: Based on physical appearance as well as chemical behaviour, manganese dioxide and copper oxide can be distinguished from each other. Copper (I) oxide (\(\left.\mathrm{Cu}_{2} \mathrm{O}\right)\) is reddish in appearance, and manganese dioxide is brown to black. Through chemical tests, these two chemicals can be easily distinguished like: When concentrated hydrochloric acid is added to manganese dioxide, it produces chlorine with greenish-yellow gas, which turns moist starch iodide paper blue-black, and copper oxide is heated with concentrated hydrochloric acid, it does not form any chlorine.
Q.2. How would you chemically distinguish \(\mathrm{MnO}_{2}\) and \(\mathrm{CuO}\) using conc. Hydrochloric acid?
Ans: When concentrated hydrochloric acid is added to manganese dioxide, it produces chlorine with greenish-yellow gas, which turns moist starch iodide paper blue-black. The chemical reaction involved in this process is \(\mathrm{MnO}_{2}+4 \mathrm{HCl} \rightarrow \mathrm{MnCl}_{2}+2 \mathrm{H}_{2} \mathrm{O}+\mathrm{Cl}_{2}\). On the other hand, when the same procedure is carried out with copper oxide, i.e., when the copper oxide is heated with concentrated hydrochloric acid, it does not form any chlorine, \(\mathrm{CuO}+\mathrm{HCl} \rightarrow \mathrm{CuCl}_{2}+\mathrm{H}_{2} \mathrm{O}\). The filtrate from manganese dioxide is brownish in colour. In contrast, the filtrate obtained from copper oxide is bluish in colour. Ammonium hydroxide \(\left(\mathrm{NH}_{4} \mathrm{OH}\right)\) to the solution of the filtrate from Manganese dioxide, no precipitate is formed, while filtrate from copper oxide gives pale blue precipitate on addition of \(\mathrm{NH}_{4} \mathrm{OH}\), and on excess \(\mathrm{NH}_{4} \mathrm{OH}\), the precipitate turns dark blue colour. This test is used to confirm the presence of copper oxide.
Q.3. How do you test for copper oxide?
Ans: There are many laboratory tests for copper oxide like When we react copper (II) oxide, which is a black solid, with colourless dilute sulphuric acid, they produce copper (II) sulphate with a characteristic blue colour. Later crystals of copper (II) sulphate pentahydrate can also be obtained. Another test is involved when the copper oxide is heated with concentrated hydrochloric acid; it does not form any chlorine; instead of chlorine, copper chloride and water molecules are formed.
Q.4. Is copper oxide a better catalyst than manganese oxide?
Ans: Copper oxides are a better catalyst than manganese oxides as they are more reactive. Copper oxides are used as a catalyst in the oxidative dehydrogenation of alcohols with air. As compared with a catalyst based on manganese oxide, copper manganese oxides show better performance for the oxidation of benzene.
Q.5. Why can manganese oxide act as a catalyst?
Ans: In the decomposition of hydrogen peroxide, manganese oxide act as a better catalyst. Manganese (IV) oxide is a catalyst that speeds up the rate of the decomposition of Hydrogen peroxide \(\left(\text {H}_{2} \text {O}_{2}\right)\) to water and oxygen gas is released in the form of bubbles. Manganese (IV) oxide will not participate in the reaction, and the reaction will remain unaffected and will remain in the solution. Manganese dioxide is also used in ferromagnetic ferrites and as a catalyst.
Q.6. How is cuprous oxide turned into copper oxide?
Ans: Crystals of cuprous oxide are found in cubic shape. When the solution of \(\mathrm{Cu}_{2} \mathrm{O}\) is heated in the presence of hydrogen. The solution is reduced very quickly. It undergoes disproportionation in the acidic solution and produces copper and copper (II) ions. On heating cupric oxide with metallic copper, it turns into cuprous oxide.
We hope this article on the Identification of Copper Oxide and Manganese Oxide has helped you. If you have any queries, drop a comment below, and we will get back to you.