- Written By
Sushmita Rout
- Last Modified 13-04-2025
Manufacturing of Sulphuric Acid by Contact Process
Manufacturing of Sulphuric Acid by Contact Process: Sulphuric acid is referred to as the king of chemicals. It is one of the most important chemical ingredients needed for the manufacture of hundreds of chemicals and many industrial processes. A nation’s industrial strength is determined by the quantity of sulphuric acid it produces and consumes. However, exposure to the acid can result in adverse health effects. Let’s understand how this acid is manufactured. In this article, we will provide all the detailed information about the Manufacturing of Sulphuric Acid by Contact Process.
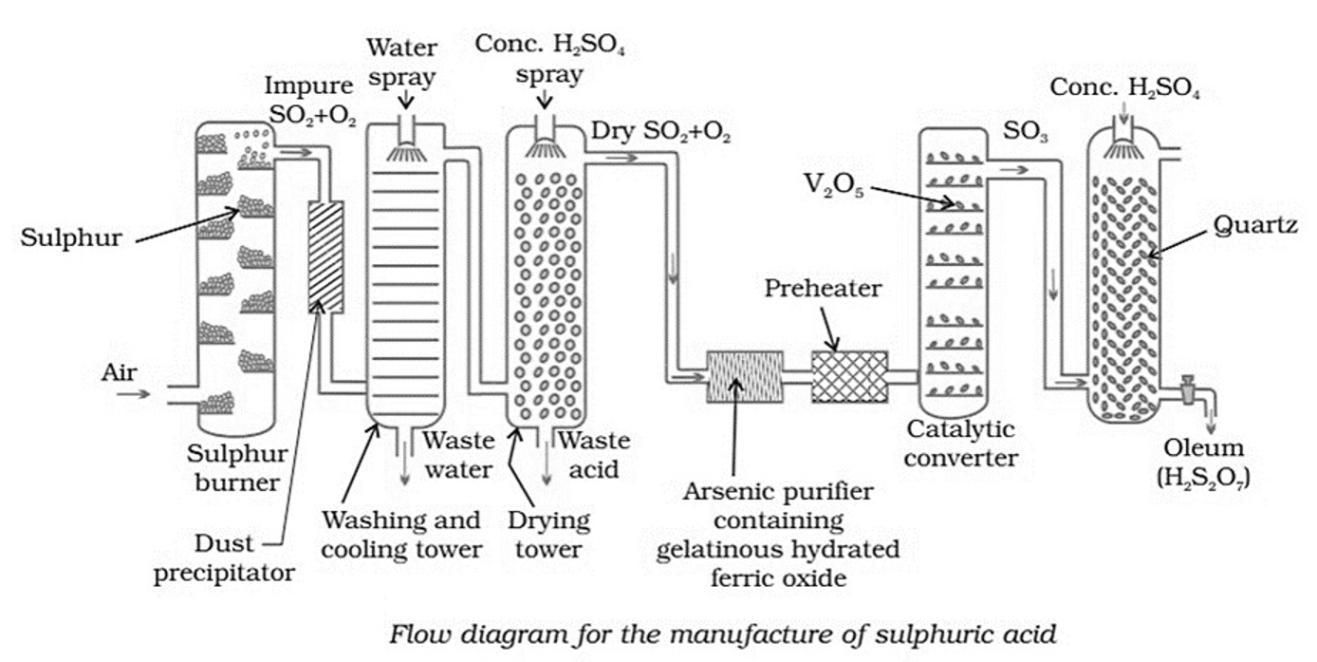
Sulphuric acid has the chemical formula \({{\text{H}}_2}{\text{S}}{{\text{O}}_4}\) and is usually manufactured by the Contact process. In this process, there are three major steps involved. These are-
(i) Production of sulphur dioxide by burning sulphur or sulphide ores in air.
(ii) Conversion of sulphur dioxide \({\text{S}}{{\text{O}}_2}\) into sulphur trioxide \({\text{S}}{{\text{O}}_3}\) by the reaction with oxygen in the presence of a catalyst \(\left( {{{\text{V}}_2}{{\text{O}}_5}} \right),\) and
(iii) absorption of \({\text{S}}{{\text{O}}_3}\) in \({{\text{H}}_2}{\text{S}}{{\text{O}}_4}\) to give oleum \(\left( {{{\text{H}}_2}{{\text{S}}_2}{{\text{O}}_7}} \right).\)
The above steps are explained in detail as below-
Production of Sulphur Dioxide by Burning of Sulphur or Sulphide Ores in Air
Sulphur burns in the presence of excess air to produce Sulphur dioxide \({\text{S}}{{\text{O}}_2}.\)
\({\text{S}}\left( {\text{s}} \right) + {{\text{O}}_2}\left( {\text{g}} \right) \to {\text{S}}{{\text{O}}_2}\left( {\text{g}} \right)\)
Conversion of Sulphur Dioxide \({\text{S}}{{\text{O}}_2}\) into Sulphur Trioxide \({\text{S}}{{\text{O}}_3}\)
Sulphur trioxide is formed when sulphur dioxide reacts with oxygen in a \(1:1\) ratio at \({400^ \circ }{\text{C – }}{450^ \circ }{\text{C}}\) and \(1 – 2\) atmospheric pressure. The reaction takes place in the presence of \({{\text{V}}_2}{{\text{O}}_5}\) as a catalyst. It is a key step in the manufacture of \({{\text{H}}_2}{\text{S}}{{\text{O}}_4}.\) This reaction is reversible in nature.
\(2{\text{S}}{{\text{O}}_2}\left( {\text{g}} \right) + {{\text{O}}_2}\left( {\text{g}} \right) \rightleftharpoons 2{\text{S}}{{\text{O}}_3}\left( {\text{g}} \right)\)
It is a reversible exothermic reaction.
The conditions needed for it are:
1. catalyst of vanadium(V) oxide, \({{\text{V}}_2}{{\text{O}}_5}\)
2. a temperature of around \(720\,{\text{K}}\)
3. a pressure of approximately \(2\) atmospheres
Low temperature and high pressure are favourable conditions as the forward reaction decreases volume.
Absorption of \({\text{S}}{{\text{O}}_3}\) in \({{\text{H}}_2}{\text{S}}{{\text{O}}_4}\) to Give Oleum \(\left( {{{\text{H}}_2}{{\text{S}}_2}{{\text{O}}_7}} \right)\)
Because sulphur trioxide is a highly exothermic process that produces a cloud of sulphuric acid, it cannot be dissolved in water directly. Hence, it is first made to react with concentrated sulphuric acid. The product so obtained is known as oleum. The oleum is then dissolved in water to obtain concentrated sulphuric acid.
\({{\text{H}}_2}{\text{S}}{{\text{O}}_4}\left( {\text{l}} \right) + {\text{S}}{{\text{O}}_3}\left( {\text{g}} \right) \to {{\text{H}}_2}\;{{\text{S}}_2}{{\text{O}}_7}\left( {\text{l}} \right)\)
\({{\text{H}}_2}\;{{\text{S}}_2}{{\text{O}}_7}\left( {\text{l}} \right) + {{\text{H}}_2}{\text{O}}\left( {\text{l}} \right) \to 2{{\text{H}}_2}{\text{S}}{{\text{O}}_4}\left( {\text{l}} \right)\)
The sulphuric acid obtained by the Contact process is \(96-98\%\) pure.
Properties
● Sulphuric acid is a colourless, dense, oily and strong mineral acid.
● It has a specific gravity of \(1.84\) at \({\text{298}}\,{\text{K}}.\)
● The acid has azing point of \(283\,{\text{k}}\) and a boiling point of \(611\,{\text{k}}.\)
● When dissolved in water, it releases a large quantity of heat.
● Slowly pour the concentrated acid into the water while stirring constantly.
● Sulphuric acid exhibits low volatility, a strong affinity for water, strong acidic character, and a strong oxidising agent.
● When dissolved in water, sulphuric acid ionises in two steps. As a result, it produces two types of salts: normal sulphates (such as sodium sulphate) and acid sulphates (such as copper sulphate) (e.g., sodium hydrogen sulphate).
\({{\text{H}}_2}{\text{S}}{{\text{O}}_4}\left( {{\text{aq}}} \right) + {{\text{H}}_2}{\text{O}}\left( {\text{l}} \right) \to {{\text{H}}_3}{{\text{O}}^ + }\left( {{\text{aq}}} \right) + {\text{HS}}{{\text{O}}_4}^ – \left( {{\text{aq}}} \right);{{\text{K}}_{{{\text{a}}_1}}} = {\text{very}}\,{\text{large}}\left( {{{\text{K}}_{{{\text{a}}_1}}} > 10} \right)\)
\({\text{HSO}}_4^ – \left( {{\text{aq}}} \right) + {{\text{H}}_2}{\text{O}}\left( {\text{l}} \right) \to {{\text{H}}_3}{{\text{O}}^ + }\left( {{\text{aq}}} \right) + {\text{SO}}_4^{2 – }\left( {{\text{aq}}} \right);{{\text{K}}_{{{\text{a}}_2}}} = 1.2 \times {10^{ – 2}}\)
● The larger value of \({{\text{K}}_{{\text{a}}1}}\left( {{{\text{K}}_{{\text{a}}1}} > 10} \right)\) shows that \({{\text{H}}_2}{\text{S}}{{\text{O}}_4}\) is largely dissociated into \({{\text{H}}^ + }\) and \({\text{HSO}}_4^ – \) ions. The strength of the acid varies directly with the value of the dissociation constant. The stronger is the acid, the greater the value of the dissociation constant \(\left( {{{\text{K}}_{\text{a}}}} \right).\)
● Due to low volatility, sulphuric acid can be used to manufacture more volatile acids from their corresponding salts.
\(2{\text{MX}} + {{\text{H}}_2}{\text{S}}{{\text{O}}_4} \to 2{\text{HX}} + {{\text{M}}_2}{\text{S}}{{\text{O}}_4}\left( {{\text{M = metal,X}} = {\text{F}},{\text{Cl}},{\text{N}}{{\text{O}}_3}} \right)\)
● Concentrated sulphuric acid is a strong dehydrating agent. The charring action on carbohydrates supports its dehydrating property.
\({{\text{C}}_{12}}{{\text{H}}_{22}}{{\text{O}}_{11}}\xrightarrow{{{{\text{H}}_2}{\text{S}}{{\text{O}}_4}}}12{\text{C}} + 11{{\text{H}}_2}{\text{O}}\)
● Hot concentrated sulphuric acid is a moderately strong oxidising agent and oxidises both metals and non-metals. In this process, it itself gets reduced to \({\text{S}}{{\text{O}}_2}.\)
\({\text{Cu}} + 2{{\text{H}}_2}{\text{S}}{{\text{O}}_4}\left( {{\text{conc}}} \right) \to {\text{CuS}}{{\text{O}}_4} + {\text{S}}{{\text{O}}_2} + 2{{\text{H}}_2}{\text{O}}\)
\({\text{S}} + 2{{\text{H}}_2}{\text{S}}{{\text{O}}_4}\left( {{\text{conc}}{\text{.}}} \right) \to 3{\text{S}}{{\text{O}}_2} + 2{{\text{H}}_2}{\text{O}}\)
\({\text{C}} + 2{{\text{H}}_2}{\text{S}}{{\text{O}}_4}\left( {{\text{conc}}.} \right) \to {\text{C}}{{\text{O}}_2} + 2{\text{S}}{{\text{O}}_2} + 2{{\text{H}}_2}{\text{O}}\)
Uses of Sulphuric Acid
● Up to \(50\) per cent of sulphuric acid is used in the production of phosphoric acid to make phosphate fertilisers.
● In the manufacture of metals such as copper, zinc etc.
● It is also used in the making of fibres.
● Used in detergent industry and as drain cleaners.
● As a catalyst in the nylon manufacturing process
● In the manufacture of \({\text{HCl,}}\) it’s used in the Manheim process.
● Used in petroleum refining
● Intermediates used in the production of pigments, paints, and dyestuffs.
● Metallurgical applications (e.g., cleansing metals before enamelling, electroplating and galvanising
● storage batteries
● As a laboratory reagent and in the production of nitrocellulose products
The industrial method of manufacturing sulfuric acid is the contact process. Sulfur dioxide and oxygen react with one other over a heated catalyst to generate sulphur trioxide, which then reacts with water to form sulfuric acid. The contact process is named after the fact that sulphur dioxide produced by the burning of sulphur ore comes in contact with the catalyst bed. In this article, we learned how the contact process is carried out in manufacturing sulphuric acid. We also learned some of the important properties of sulphuric acid along with its uses.
Q.1. How is Sulphuric acid manufactured by the contact process?
Ans: In the contact process, sulphur combines with oxygen to form sulphur dioxide that further gets converted into sulphur trioxide. Sulphur trioxide then combines with sulphuric acid to form oleum which then reacts with water to form sulphuric acid.
Q.2. What are the steps of the contact process?
Ans: (i) Production of sulphur dioxide by burning sulphur or sulphide ores in air.
(ii) Conversion of sulphur dioxide \({\text{S}}{{\text{O}}_2}\) into sulphur trioxide \({\text{S}}{{\text{O}}_3}\) by the reaction with oxygen in the presence of a catalyst \(\left( {{{\text{V}}_2}{{\text{O}}_5}} \right),\) and
(iii) Absorption of \({\text{S}}{{\text{O}}_3}\) in \({{\text{H}}_2}{\text{S}}{{\text{O}}_4}\) to give oleum \(\left( {{{\text{H}}_2}{{\text{S}}_2}{{\text{O}}_7}} \right).\)
Q.3. Why is the manufacture of Sulphuric acid called the contact process?
Ans: The \({\text{S}}{{\text{O}}_2}\) evolved from the burning of sulfur ore comes in contact with catalyst bed; hence the name of this process is called contact process.
Q.4. Which catalyst is used in the contact process?
Ans: The catalyst used in the contact process is vanadium pentoxide.
Q.5. What is sulphuric acid used for?
Ans: Sulphuric acid is used in the manufacture of fertilisers, pigments, dyes, drugs, explosives, detergents, inorganic salts and acids, as well as in petroleum refining and metallurgical processes.
We hope this article on the Manufacturing of Sulphuric Acid by Contact Process has helped you. If you have any queries, drop a comment below, and we will get back to you.