- Written By
Ankita Sahay
- Last Modified 13-01-2025
Measurement of Energy Changes: Calorimetry
Measurement of Energy Changes – Calorimetry: We all know the fact that energy can neither be created nor be destroyed. Every substance in this universe is made up of atoms and molecules that are continuously moving around and collide with each other. Due to this movement or collision, atoms or molecules release energy in the form of heat. This is one of the basics of the principles of calorimetry. Thermal energy is present everywhere i.e., in the human body, in volcanoes and even in the coolest places. Heat energy is transferable from one body to another body. The flow of heat is involved in the physical processes and chemical reactions which is measurable. The process by which heat is measured is known as calorimetry. The word calorimetry is derived from ‘calories’ which is the unit of energy. Let’s have a look at the measurement of energy changes in detail through this article.
Definition of Calorimetry
Calorimetry can be defined as an act of measuring the change in the thermal energy of an object. Heat change is associated with changes of its state, for example, changes to Chemical reaction, physical changes, or transition of phases under specified conditions. Calorimetry is usually performed with a device named a calorimeter. The word ‘calorimetry’ is derived from the Latin word ‘calor’, which means heat and the Greek word (metron), which means to measure. The first scientist to recognise the difference between heat and temperature was Joseph Black. He was the first founder of the science of calorimetry.
Some of the facts related to calorimetry are as follows:
1. The amount of heat present in the body is determined by the temperature of the body.
2. Greater is the amount of heat energy, more is the temperature of a body. Thus, it can be said that the temperature and heat energy are directly proportional to each other.
3. To measure the loss and gain of thermal energy, the temperature of an object is measured before and after the transfer of heat. With the help of this temperature difference, the heat change of a body can be determined.
Example of Energy Changes
When we cook food in a metal utensil, slowly its handle becomes hot because the heat gets transferred to different parts of the utensil.
Another example is a cup of hot coffee or chilled ice cream kept at room temperature. After some time, we notice that the coffee will cool down, and the ice cream will melt. This change occurs because the temperature of the coffee is reduced as it releases heat energy. On the other hand, the temperature of ice cream rises as it absorbs heat from the atmosphere.
The principle of calorimetry is to make a significant measurement of the amount of heat energy transferred in a system and its relation to the temperature. In a calorimeter, two types of matter like mostly a liquid and a solid are present in contact with one another. Both the bodies have definite temperatures. Thus, heat energy gets transferred from the object having greater temperature to the object having lesser temperature as a result of this type of arrangement. The heat energy flow continues until a state of thermal equilibrium is achieved between the two bodies. The principle of calorimetry designates the “law of conservation of energy” which means ‘energy can neither be formed nor destroyed’, it can only be converted from one form to another form’. Hence, according to this statement, the total amount of heat energy absorbed by the cold object is equal to the total amount of heat energy released by the hot object.
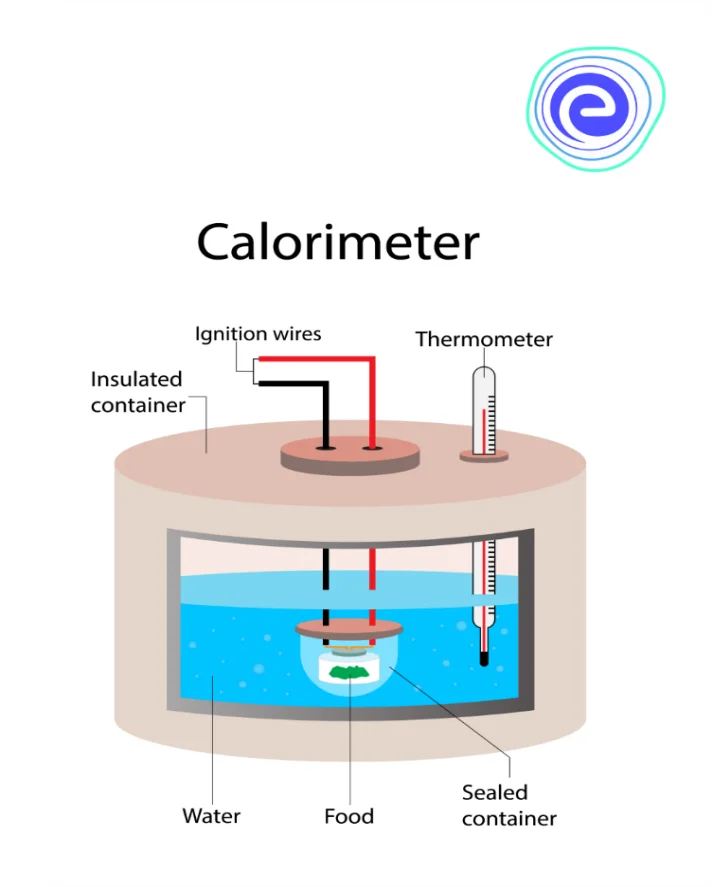
What is a Calorimeter?
Calorimetry is the process by which the heat of a chemical reaction or the physical changes, as well as the heat capacity, is measured with the help of a device named a ‘calorimeter’.
A simple calorimeter consists of a thermometer that is attached to a metal container full of water that is suspended above the combustion chamber. In this calorimeter, to find the heat change of a substance, the sample is added to a calorimeter and the initial and the final temperature of the reaction is noted and recorded. Finally, the temperature change is multiplied by the mass and thus, the specific heat capacity of the substance gives a value for the energy released or absorbed during the reaction.
Heat Capacity of Calorimeter
The quantity of heat absorbed by the calorimeter for every \({{\rm{1}}^{\rm{o}}}{\rm{C}}\) rise in temperature is known as the heat capacity of the calorimeter.
According to the basic concept of calorimetry:
Amount of heat released by hot object \(=\) Amount of heat absorbed by cold object
The transfer of heat energy is calculated with the help of a formula given below:
\({\rm{Q = mC\Delta T}}\)
In the above equation,
- \({\rm{Q}}\) is the heat or energy change. If the value of \({\rm{Q}}\) is positive, then the heat is absorbed by the system and the reaction is endothermic. On the other hand, if the value of \({\rm{Q}}\) is negative, then the heat is released by the system and the reaction is exothermic. \({\rm{Q}}\) is measured in terms of Joules.
- \({\rm{m = }}\) The mass of the sample taken. It is measured in grams.
- \({\rm{C}}\) is the specific heat of the substance which is a constant.
- \({\rm{\Delta T}}\) is the change in the temperature.
Different Types of Calorimeters
Different types of calorimeters are as follows:
- Reaction Calorimeters
- Adiabatic Calorimeters
- Constant Pressure Calorimeters
- Bomb Calorimeters (Constant Volume Calorimeters)
- Differential Scanning Calorimeters
Important Questions on Measurement of Energy Changes: Calorimetry
Summary of Measurement of Energy Changes
In a nutshell, the Law of conservation of Energy states that the energy can neither be formed nor be destroyed; it can only be converted from one form to another form’. The calorimeter is a device that measures the change in energy of a system. The working of the calorimeter is based on this principle. Calorimetry is the part of science that is related to the measurement of the state of a body with respect to heat energy in order to measure its physical and chemical changes. Physical changes involve melting, evaporation, etc. In contrast, chemical changes include burning, acid-base reaction, neutralisation reaction, etc. A simple calorimeter consists of a thermometer that is attached to a metal container full of water that is suspended above the combustion chamber. The quantity of heat absorbed by the calorimeter for every \({{\rm{1}}^{\rm{o}}}{\rm{C}}\) rise in temperature is known as the heat capacity of the calorimeter. The formula of the heat capacity is \({\rm{Q = mC\Delta T}}\). Thus, the flow of heat can be determined by using a calorimeter.
FAQs on Measurement of Energy Changes
Q.1. What is the definition of calorimetry?
Ans. Calorimetry is defined as an act of measuring the change in the thermal energy of an object. Heat change is associated with changes in its state, for example, changes to chemical reactions, physical changes, or transition of phases under specified conditions. Calorimetry is usually performed with a device named a calorimeter. The word ‘calorimetry’ is derived from the Latin word ‘calor’, which means heat and the Greek word (metron), which means to measure.
Q.2. What measurements are needed for calorimetry?
Ans. In calorimetry, the quantity of heat absorbed by the calorimeter for every \({{\rm{1}}^{\rm{o}}}{\rm{C}}\) rise is measured. This heat change is measured with the help of a device named a calorimeter. A simple calorimeter consists of a thermometer that is attached to a metal container that is full of water that is suspended above the combustion chamber. In order to find the heat change of a substance, the sample is added to a calorimeter, and the initial and the final temperature of the reaction is noted. Finally, the temperature change is multiplied by the mass, and thus, the specific heat capacity of the substance gives a value for the energy released or absorbed during the reaction taking place.
Q.3. How do you measure the change in energy?
Ans. The change in energy is measured or calculated with the help of a formula: \({\rm{Q = mC\Delta T}}\) In this equation, \({\rm{Q}}\) is the heat or energy change. If the value of \({\rm{Q}}\) is positive, then the heat is absorbed by the system, and the reaction is endothermic. On the other hand, if the value of \({\rm{Q}}\) is negative, then the heat is released by the system, and the reaction is exothermic. \({\rm{Q}}\) is measured in terms of Joules. ‘\({\text{m}}\)’ is the mass of the sample taken, measured in grams. \({\rm{C}}\) is the specific heat of the substance, which is a constant, and \(\Delta {\rm{T}}\) is the change in the temperature.
Q.4. What are the principles of calorimetry?
Ans. The principle of calorimetry deals with the significant measurement of the amount of heat energy transferred in a system and its relation to the temperature. In a calorimeter, mostly a liquid and a solid are present in contact with one another having definite temperatures. Thus, heat energy gets transferred from the object having greater temperature to the object having a lesser temperature. The heat energy flow continues until a state of thermal equilibrium is achieved between the two bodies. The principle of calorimetry designates the “law of conservation of energy” which means ‘energy can neither be formed nor destroyed, it can only be converted from one form to another form’.
Q.5. What is the instrument used to measure heat?
Ans. The instrument used to measure heat is known as a calorimeter. It consists of a thermometer that is attached to a metallic container full of water that is suspended above the combustion chamber.
Learn Everything About Flame Here
We hope this article on Measurement of Energy Changes: Calorimetry has helped you. If you have any queries, drop a comment below, and we will get back to you.