- Written By
Ankita Sahay
- Last Modified 08-04-2025
Nitro Compounds: Types, Synthesis, Properties and Uses
Nitro compounds are a group of organic compounds having Nitro group \({\rm{( – O – N = O)}}\) as a part of its molecular structure. Nitro compounds are polar in nature that appear as pale yellow or colourless liquids and are slightly soluble in water. A Nitro compound is prepared by the reaction, called Nitration. It takes place between nitric acid and an organic compound. Aromatic compounds, such as benzene or toluene, undergoes Nitration by treating them with a mixture of nitric acid and sulphuric acids at a high temperature of about \({\rm{10}}{{\rm{0}}^{\rm{o}}}{\rm{C}}\).
At the same time, Nitration of aliphatic compounds needs a higher temperature of about \({\rm{40}}{{\rm{0}}^{\rm{o}}}{\rm{C}}\). Different types of compounds are formed by Nitrogen, such as ammonia, nitrous oxides, cyanide, etc. In nature, complex compounds of nitrogen complexes are formed by nitrification and de-nitrification. In this process, nitrogen-fixing bacterias present within the soil convert ammonia into nitrite. Which, in turn, gets converted into nitrates. These complex compounds are taken up by the plants and utilised in forming animal and plant proteins. In this article, we will focus on Nitro compounds.
Definition of Nitro Compounds
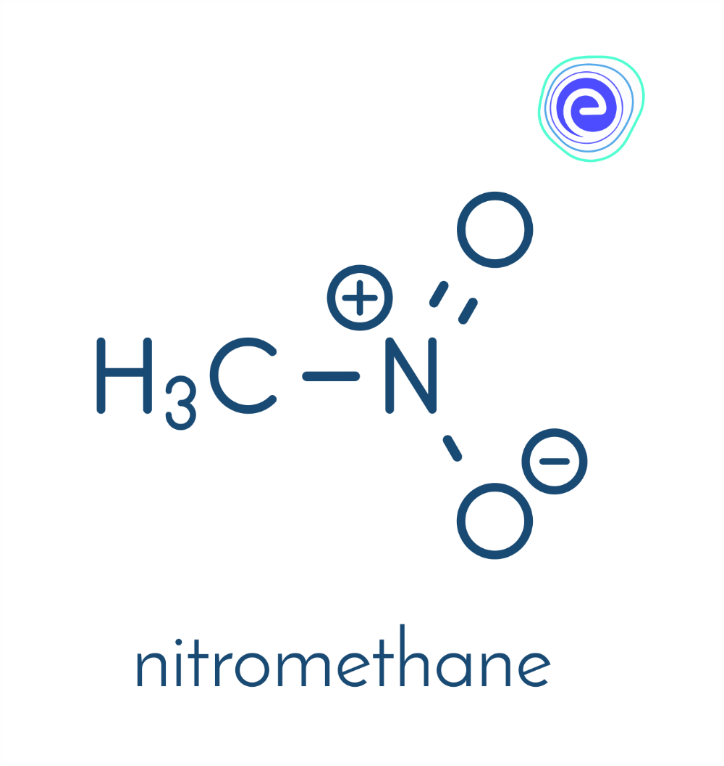
Organic compounds that contain one or more Nitro functional groups \(\left(-\mathrm{NO}_{2}\right)\). It is a strong electron-withdrawing group. Due to this property, alpha \({\rm{C – H}}\) bonds present adjacent to the Nitro group is acidic. For this reason, the presence of nitro groups in aromatic compounds slows down electrophilic aromatic substitution but facilitates nucleophilic aromatic substitution. Nitro compounds are one of the most common functional group that makes a compound explosive and is used globally. Nitro groups are rarely found in nature and are mostly produced by nitration reactions starting with nitric acid.
Physical Properties of Nitro Compounds
- Nitroalkanes are colourless, pleasant-smelling liquids.
- Aromatic nitro compounds can exist as either pale yellow liquids or solids with distinctive smells. For example, Nitrobenzene is a pale yellow liquid that has the smell of bitter almonds.
- Nitro compounds have a high boiling point because both nitroalkanes and nitroarenes are highly polar compounds and have strong dipole-dipole interactions. Due to this reason, nitro compounds have a high boiling point.
- Nitroalkanes are slightly soluble in water, while nitroarenes are insoluble.
Synthesis of Nitro Compounds
Aromatic Nitro Compounds
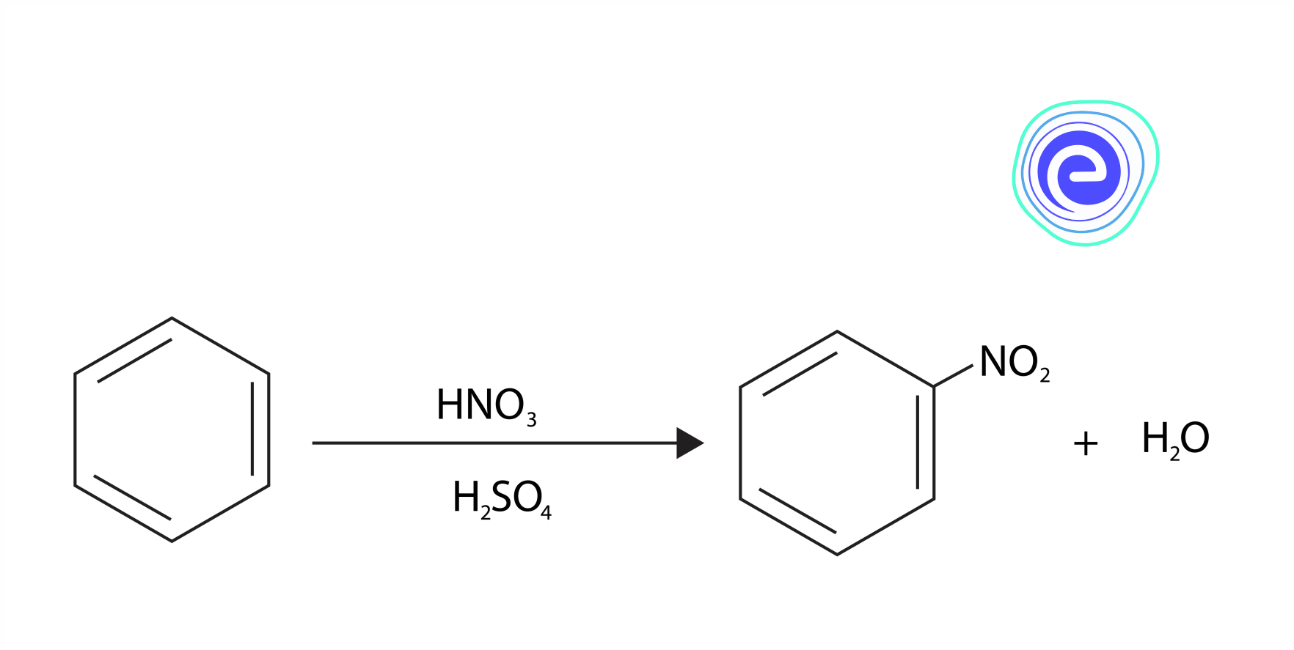
Nitro compounds are synthesised by the process of ‘Nitration’. In this process, nitric acid and sulphuric acid are mixed, which produces an electrophile, i.e., Nitronium ion \(\left(\mathrm{NO}_{2}^{+}\right)\). Nitrobenzene is formed by the Nitration of benzene, and this is the most commercially used Nitro compound. Picric acid, which is chemically known as trinitrophenol, is widely used for making explosives. The chemical reaction for the Nitration of benzene to Nitrobenzene is given below:
Aliphatic Nitro Compounds
Aliphatic Nitro compounds are prepared from hydrocarbons by the vapour phase nitration method. In this method, a hydrocarbon is heated at a high temperature in the presence of fuming nitric acid to produce the nitro compound.
\({\text{C}}{{\text{H}}_3} + {\text{HN}}{{\text{O}}_3}\left( {{\text{fuming}}} \right)\xrightarrow{{{\text{730}}\,{\text{K}}}}{\text{C}}{{\text{H}}_{\text{3}}}{\text{N}}{{\text{O}}_{\text{2}}} + {{\text{H}}_{\text{2}}}{\text{O}}\)
Classification of Nitro Compounds
Nitro compounds are classified based on the type of carbon to which it is attached.
- Primary Nitroalkanes: The nitro group that is attached to a primary carbon atom is known as the primary nitroalkane. For example \({\rm{RC}}{{\rm{H}}_{\rm{2}}}{\rm{N}}{{\rm{O}}_{\rm{2}}}\)
- Secondary Nitroalkanes: The nitro group that is attached to a secondary carbon atom is known as the secondary nitroalkane. For example: \({{\rm{R}}_{\rm{2}}}{\rm{CHN}}{{\rm{O}}_{\rm{2}}}\)
- Tertiary Nitroalkanes: The nitro group that is attached to a tertiary carbon atom is known as the tertiary nitroalkane. For example: \({{\rm{R}}_{\rm{3}}}{\rm{CN}}{{\rm{O}}_{\rm{2}}}\)
Stability of Nitro Compounds
- Nitroalkanes are mostly stable and hence, can be distilled without decomposition under atmospheric pressure.
- Nitroarenes cannot be distilled under atmospheric pressure as compared to nitroalkanes since aromatic nitro compounds may either decompose or explode on strong heating.
- Many aromatic nitro compounds are volatile and hence, can be easily purified by steam distillation.
Uses of Nitro Compounds
- Nitro compounds such as nitroalkanes, for example, nitromethane, nitroethane etc., and Nitrobenzene are widely used as solvents in industry.
- In the manufacture of detergents, dyes, and pharmaceuticals, Nitroarenes are important intermediates.
- Nitro compounds are used as explosives like \(2,4,6\)-Trinitrotoluene (TNT).
- Nitroalkanes are also used as propellants in the industry.
- Nitro compounds such as Chloropicrin is used as a fumigant to control pests in the soil in agricultural areas and as a constituent of tear gas.
Summary
Organic compounds that contain one or more Nitro functional groups \(\left( {{\rm{ – N}}{{\rm{O}}_{\rm{2}}}} \right)\). It is a strong electron-withdrawing group. Nitro compounds are polar in nature that appear as pale yellow or colourless liquids and are slightly water-soluble. Nitro compounds are prepared by the reaction, called Nitration. It is a strong electron-withdrawing group. Due to this property, alpha \({\rm{C – H}}\) bonds present adjacent to the Nitro group behaves as an acid. It takes place between nitric acid and an organic compound. Aromatic compounds, such as benzene or toluene, undergoes Nitration by treating them with a mixture of nitric acid and sulphuric acids at a high temperature of about \({\rm{10}}{{\rm{0}}^{\rm{^\circ }}}{\rm{C}}\). Whereas Nitration of aliphatic compounds needs a higher temperature of about \({\rm{40}}{{\rm{0}}^{\rm{^\circ }}}{\rm{C}}\).
Different types of compounds are formed by Nitrogen, such as ammonia, nitrous oxides, cyanide, etc. In nature, complex compounds of nitrogen complexes are formed by nitrification and de-nitrification. Nitroalkanes are colourless, pleasant-smelling liquids. Aromatic nitro compounds can exist as either pale yellow liquids or solids with distinctive smells. For example, Nitrobenzene is a pale yellow liquid that has the smell of bitter almonds. These compounds have a high boiling point because both nitroalkanes and nitroarenes are highly polar compounds and have strong dipole-dipole interactions. Due to this reason, nitro compounds have a high boiling point. Nitroalkanes are slightly soluble in water, while nitroarenes are insoluble.
Different types of nitro compounds include Primary Nitroalkanes: The nitro group that is attached to a primary carbon atom is known as the primary nitroalkane. For example \({\rm{RC}}{{\rm{H}}_{\rm{2}}}{\rm{N}}{{\rm{O}}_{\rm{2}}}\). Secondary Nitroalkanes: The nitro group that is attached to a secondary carbon atom is known as the secondary nitroalkane. For example: \({{\rm{R}}_{\rm{2}}}{\rm{CHN}}{{\rm{O}}_{\rm{2}}}\). Tertiary Nitroalkanes: The nitro group that is attached to a tertiary carbon atom is known as the tertiary nitroalkane. For example: \({{\rm{R}}_{\rm{3}}}{\rm{CN}}{{\rm{O}}_{\rm{2}}}\). Thus, in this article, we have learnt about various nitro compounds and their uses at the industrial level. These are very important chemical compounds.
FAQs on Nitro Compounds
Q.1. What are nitro compounds? Explain with the help of an example.
Ans: Organic compounds that contain one or more Nitro functional groups \(\left( {{\rm{ – N}}{{\rm{O}}_{\rm{2}}}} \right)\). It is a strong electron-withdrawing group. Due to this property, alpha \({\rm{C – H}}\) bonds present adjacent to the Nitro group is acidic. For this reason, the presence of nitro groups in aromatic compounds slows down electrophilic aromatic substitution but facilitates nucleophilic aromatic substitution. Nitro compounds are one of the most common functional group that makes a compound explosive and is used globally. Nitro groups are rarely found in nature and are mostly produced by nitration reactions starting with nitric acid. Some examples of nitro compounds are nitromethane, nitrobenzene, nitrotoluene, etc.
Q.2. How are organic compounds with nitro functional groups obtained?
Ans: Nitro compounds are obtained by replacing one or more hydrogen atoms in the hydrocarbon with a nitro group \(\left( {{\rm{ – N}}{{\rm{O}}_{\rm{2}}}} \right)\). In nitro compounds, the Nitrogen is directly attached to the carbon atom in the organic compound. Aliphatic Nitro compounds are prepared from hydrocarbons by the vapour phase nitration method. In this method, a hydrocarbon is heated at a high temperature in the presence of fuming nitric acid to produce the nitro compound. The chemical reaction accompanied in this process includes:
\({\rm{C}}{{\rm{H}}_3}{\rm{H}} + {\rm{HN}}{{\rm{O}}_3}{\rm{(fuming)}}730\;{\rm{K}} \to {\rm{C}}{{\rm{H}}_3}{\rm{N}}{{\rm{O}}_2} + {{\rm{H}}_2}{\rm{O}}\)
Q.3. How are Nitro compounds classified?
Ans: Nitro compounds are classified based on the type of carbon to which it is attached. They are (i) Primary Nitroalkanes: The nitro group that is attached to a primary carbon atom is known as the primary nitroalkane. For example \({\rm{RC}}{{\rm{H}}_{\rm{2}}}{\rm{N}}{{\rm{O}}_{\rm{2}}}\). (ii) Secondary Nitroalkanes: The nitro group that is attached to a secondary carbon atom is known as the secondary nitroalkane. For example: \({{\rm{R}}_{\rm{2}}}{\rm{CHN}}{{\rm{O}}_{\rm{2}}}\). (iii) Tertiary Nitroalkanes: The nitro group that is attached to a tertiary carbon atom is known as the tertiary nitroalkane. For example: \({{\rm{R}}_{\rm{3}}}{\rm{CN}}{{\rm{O}}_{\rm{2}}}\)
Q.4. What are the physical properties of Nitro compounds?
Ans: Nitroalkanes are colourless, pleasant-smelling liquids. Aromatic nitro compounds can exist as either pale yellow liquids or solids with distinctive smells. For example, Nitrobenzene is a pale yellow liquid that has the smell of bitter almonds. Nitro compounds have a high boiling point because both nitroalkanes and nitroarenes are highly polar compounds and have strong dipole-dipole interactions. Due to this reason, nitro compounds have a high boiling point. Nitroalkanes are slightly soluble in water, while nitroarenes are insoluble.
Q.5. How are nitro compounds reduced?
Ans: Nitro compounds \(\left(\mathrm{RNO}_{2}\right)\) are reduced by hydrogen or other reducing agents. Primary nitro compounds are reduced to primary amines. Amines are widely used in making fertilisers which are widely used in agriculture.
Q.6. What makes a nitro compound an explosive compound?
Ans: The addition of more nitro groups in a compound makes it more explosive. For example, \(2,4,6\)-Trinitrotoluene (TNT) is a widely used explosive due to the presence of three nitro groups. The reason due to which nitro compounds form explosives is because of the high electron-withdrawing character.
Q.7. Are Nitro compounds stable?
Ans: Nitroalkanes are mostly stable and hence, can be distilled without decomposition under atmospheric pressure. Nitroarenes cannot be distilled under atmospheric pressure as compared to nitroalkanes since aromatic nitro compounds may either decompose or explode on strong heating. Many aromatic nitro compounds are volatile and hence, can be easily purified by steam distillation.
We hope this article on Nitro Compounds has helped you. If you have any queries, drop a comment below, and we will get back to you.