- Written By
Paramjit Singh
- Last Modified 31-03-2025
Occurrence of Group 17 Elements: Melting & Boiling Points, Electronegativity
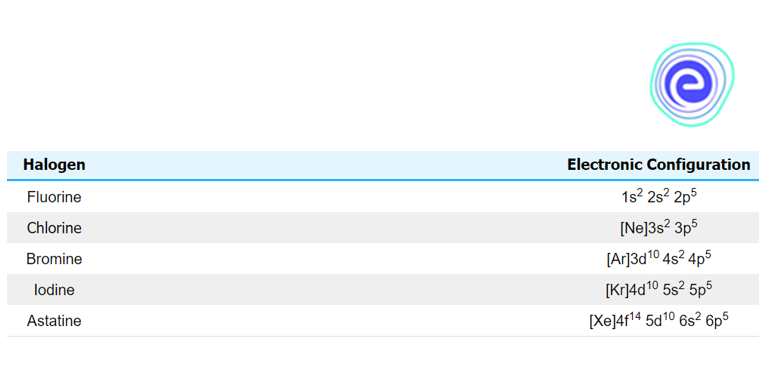
Occurrence of Group 17 Elements: On the periodic table, the halogens are to the left of the noble gases. Fluorine \(\left( {\rm{F}} \right){\rm{,}}\) chlorine \(\left( {{\rm{Cl}}} \right){\rm{,}}\) bromine \(\left( {{\rm{Br}}} \right){\rm{,}}\) iodine \(\left( {\rm{I}} \right){\rm{,}}\) and astatine \(\left( {{\rm{At}}} \right)\) are the five poisonous non-metallic elements that make up Group \(17\) of the periodic table \(\left( {{\rm{At}}} \right){\rm{.}}\) Despite the fact that astatine is radioactive and has only short-lived isotopes, it behaves similarly to iodine and is frequently classified as a halogen. Because halogen elements have seven valence electrons, forming a full octet requires only one extra electron. Because of this, they are more reactive than other non-metal groups.
In their elemental states, halogens form diatomic molecules (of the form \({{\rm{X}}_2},\) where \({\rm{X}}\) signifies a halogen atom). These diatomic compounds have non-polar covalent single bonds as their bonds. On the other hand, Halogens quickly mix with most elements and are never seen in nature uncombined. Fluorine is the most reactive halogen in general, while astatine is the least reactive. Group \(1\) salts are formed by all halogens and have comparable characteristics. Halogens are found as halide anions with a charge of \(-1\) in these compounds (e.g. \({{\rm{C}}{{\rm{l}}^ – },}\) \({{\rm{B}}{{\rm{r}}^ – },}\) etc.). The presence of halide anions is indicated by replacing the -ine ending with a -ide ending; for example, \({{\rm{C}}{{\rm{l}}^ – }}\) is termed “chloride.”
Occurrence Of Group 17 Elements: Overview
Fluorine – Fluorine is a chemical element with the atomic number \(9\) and the symbol \({{\rm{F}}{\rm{.}}}\) In \(1886,\) the element fluorine was isolated from hydrofluoric acid for the first time. Fluorine is the most abundant halogen in the Earth’s crust and exists as a diatomic molecule in its state \(\left( {{{\rm{F}}_2}} \right).\) Fluorine is the periodic table’s most electronegative element. At room temperature, it appears as a pale-yellow gas. Fluorine’s atomic radius is also quite modest. Except in its elemental, diatomic state, its oxidation state is always \(-1.\) (in which its oxidation state is zero). Except for helium \(\left( {{\rm{He}}} \right){\rm{,}}\) neon \(\left( {{\rm{Ne}}} \right){\rm{,}}\) and argon, fluorine is a highly reactive element that reacts directly with all other elements \(\left( {{\rm{Ar}}} \right){\rm{.}}\)
Chlorine – Chlorine is a chemical element with the atomic number \(17\) and the symbol \({{\rm{Cl}}{\rm{.}}}\) Hydrochloric acid was used to extract chlorine, which was discovered in \(1774.\) It produces the diatomic molecule \({{\rm{C}}{{\rm{l}}_2}}\) in its elemental state. Chlorine exists in a variety of oxidation states, including \(-1, +1, 3, 5,\) and \(7.\) It appears as a light green gas at normal temperature. The \({{\rm{C}}{{\rm{l}}_2}}\) molecule is extremely reactive due to the weak link that develops between the two chlorine atoms. Chlorine combines with metals to form chlorides, which are salts. The most common ions that dissolve in the ocean are chloride ions. There are two isotopes of chlorine: \(^{{\rm{35}}}{\rm{Cl}}\) and \(^{{\rm{35}}}{\rm{Cl}}.\) The chlorides’ most common chemical is sodium chloride.
Bromine – Bromine is a chemical element with the symbol \({\rm{Br}}\) and an atomic number of \(35.\) It was discovered for the first time in \(1826.\) It is the diatomic molecule \({\rm{B}}{{\rm{r}}_2}\) in its elemental form. Bromine is a reddish-brown liquid at ambient temperature. Its oxidation states range from \(-1\) to \(+1, 3, 4,\) and \(5,\) respectively. Bromine has higher reactivity than iodine but is not as high as chlorine. Bromine is also divided into two isotopes: \(^{{\rm{79}}}{\rm{Br}}\) and \(^{{\rm{81}}}{\rm{Br}}{\rm{.}}\) Bromine is made up of bromide salts that are present in the sea.
Iodine – Iodine is a chemical element with the atomic number \(53\) and the symbol \({\rm{I}}.\) The oxidation states of iodine are \(-1, +1, 5,\) and \(7.\) In its elemental state, iodine occurs as a diatomic molecule, \({{\rm{I}}_2}.\) It appears as a violet solid at normal temperature. The stable isotope of iodine is \(^{127}{\rm{I}}{\rm{.}}\) It was first found in \(1811\) with the use of sulfuric acid and seaweed. Iodide ions can currently be extracted from salt water. Although iodine is not highly soluble in water, if specific iodides are added to the solution, the solubility may rise. Thyroid hormone production is one of the many crucial functions of iodine in life.
Astatine – Astatine is a radioactive element with the symbol At and an atomic number of \(85.\) The following oxidation states are possible: \(-1, +1, 3, 5,\) and \(7.\) At ambient temperature, it appears as a black, metallic solid and is the only halogen that is not a diatomic molecule. Astatine is an extremely rare element. Hence there isn’t a lot of information about it. Furthermore, astatine has a relatively short radioactive half-life of only a few hours. Synthesis led to its discovery in \(1940.\) Also, astatine is supposed to be comparable to iodine. The metallic quality of these two elements, on the other hand, is thought to distinguish them.
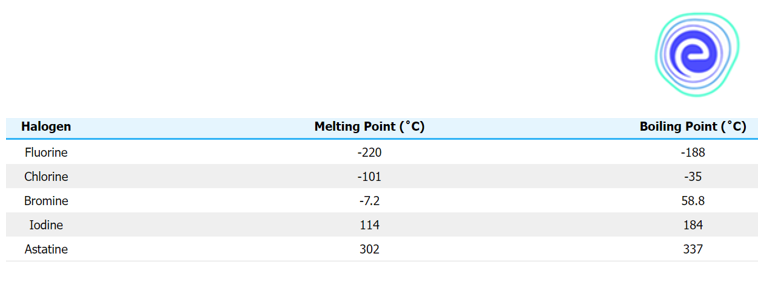
Melting and Boiling Points of Group 17 Elements
The van der Waals forces cause the melting and boiling points to rise as you go down the group. The size of the molecules gets bigger as you go down the line. This increase in size means an increase in the strength of the van der Waals forces.
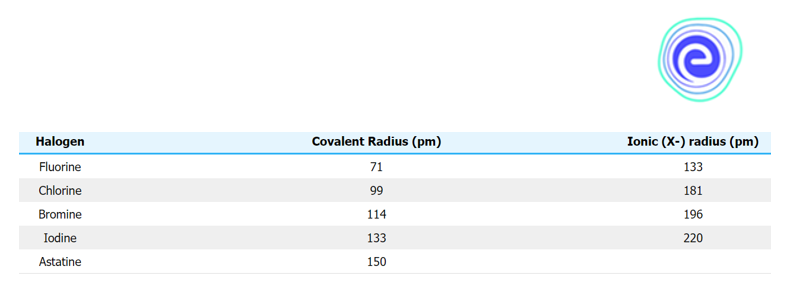
Atomic Radius of Group 17 Elements
Because the number of protons and neutrons rises, the size of the nucleus increases down a group \(({\rm{F}} < {\rm{Cl}} < {\rm{Br}} < {\rm{I}} < {\rm{At}}).\) In addition, with each period, more energy levels are added. As a result, the orbital is larger, resulting in a larger atomic radius.
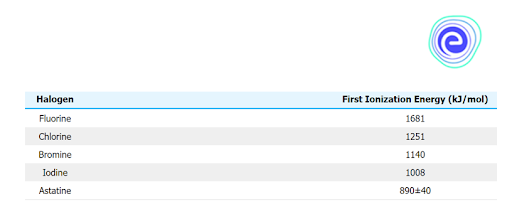
Ionisation Energy
It takes less energy to remove outer valence electrons if they are not close to the nucleus. As a result, because there are more energy levels at the bottom of the group, the energy required to take off the outermost electron is lower. The high ionisation energy also gives the element a non-metallic appearance. Because iodine and astatine have metallic qualities, the ionisation energy lowers as one moves along the group \(({\rm{At}} < {\rm{l}} < {\rm{Br}} < {\rm{Cl}} < {\rm{F}}).\)
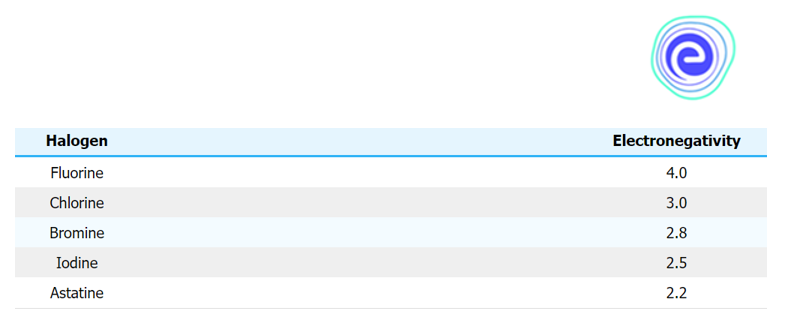
Electronegativity
Due to the increase in energy levels at increasingly lower levels, the number of valence electrons in an atom increases along with the group. Because the electrons are moving away from the nucleus, the nucleus and electrons are becoming less attracted to each other. Shielding appears to be increasing. As a result, electronegativity diminishes as one moves down the group \(({\rm{At}} < {\rm{l}} < {\rm{Br}} < {\rm{Cl}} < {\rm{F}}).\)
Electron Affinity
Electron affinity normally diminishes as atomic size lowers down the group \(({\rm{At}} < {\rm{l}} < {\rm{Br}} < {\rm{F}} < {\rm{Cl}}).\) The nucleus will be less attractive to an electron, resulting in a reduced electron affinity. Fluorine, on the other hand, has a lower electron affinity than chlorine. This is due to fluorine’s smaller size when compared to chlorine.
Summary
On the periodic table, the halogens are to the left of the noble gases. Fluorine \(\left( {\rm{F}} \right){\rm{,}}\) chlorine \(\left( {{\rm{Cl}}} \right){\rm{,}}\) bromine \(\left( {{\rm{Br}}} \right){\rm{,}}\) iodine \(\left( {\rm{I}} \right){\rm{,}}\) and astatine \(\left( {\rm{A}} \right)\) are the five poisonous non-metallic elements that makeup Group \(17\) of the periodic table \(\left( {{\rm{At}}} \right).\) Fluorine is a chemical element with the atomic number \(9\) and the symbol \({\rm{F}}{\rm{.}}\) In \(1886,\) the element fluorine was isolated from hydrofluoric acid for the first time. Fluorine is the most abundant halogen in the Earth’s crust and exists as a diatomic molecule in its state \(\left( {{{\rm{F}}_{\rm{2}}}} \right){\rm{.}}\)
Chlorine is a chemical element with the atomic number \(17\) and the symbol \(\left( {{\rm{Cl}}} \right).\) Hydrochloric acid was used to extract chlorine, which was discovered in \(1774.\) It produces the diatomic molecule \({{\rm{C}}{{\rm{l}}_2}}\) in its elemental state. Chlorine exists in a variety of oxidation states, including \(-1, +1, 3, 5,\) and \(7.\) It appears as a light green gas at normal temperature. Bromine is a chemical element with the symbol \({\rm{Br}}\) and an atomic number of \(35.\) It was discovered for the first time in \(1826.\) It is the diatomic molecule \({\rm{B}}{{\rm{r}}_2}\) in its elemental form. In its elemental state, iodine occurs as a diatomic molecule, \({{\rm{I}}_{\rm{2}}}{\rm{.}}\) It appears as a violet solid at normal temperature.
FAQs on Occurrence of Group 17 Elements
Q.1. Discuss the occurrence and oxidation state of fluorine.
Ans: Fluorine is a chemical element with the atomic number \(9\) and the symbol \({\rm{F}}{\rm{.}}\) In \(1886,\) the element fluorine was isolated from hydrofluoric acid for the first time. Fluorine is the most abundant halogen in the Earth’s crust and exists as a diatomic molecule in its state \(\left( {{{\rm{F}}_{\rm{2}}}} \right).\) Fluorine is the periodic table’s most electronegative element. At room temperature, it appears as a pale-yellow gas. Fluorine’s atomic radius is also quite modest. Except in its elemental, diatomic state, its oxidation state is always \(-1.\)
Q.2. Discuss the melting and boiling point of group 17 elements.
Ans: The van der Waals forces cause the melting and boiling points to rise as you go down the group. The size of the molecules gets bigger as you go down the line. This increase in size means an increase in the strength of the van der Waals forces.
Q.3. What is the trend of atomic radius for group 7 elements?
Ans: Because the number of protons and neutrons rises, the size of the nucleus increases down a group \(({\rm{F}} < {\rm{Cl}} < {\rm{Br}} < 1 < {\rm{At}}).\) In addition, with each period, more energy levels are added. As a result, the orbital is larger, resulting in a larger atomic radius.
Q.4. Which group 17 element has the highest ionisation energy?
Ans: Fluorine has the highest ionisation energy in group 17.
Q.5. What is the trend of electronegativity for group 17 elements?
Ans: Due to the increase in energy levels at increasingly lower levels, the number of valence electrons in an atom increases along with the group. Because the electrons are moving away from the nucleus, the nucleus and electrons are becoming less attracted to each other. Shielding appears to be increasing. As a result, electronegativity diminishes as one moves down the group \(({\rm{At}} < {\rm{l}} < {\rm{Br}} < {\rm{Cl}} < {\rm{F}}).\)
We hope this article on the Occurrence of Group 17 Elements has helped you. If you have any queries, drop a comment below, and we will get back to you.