- Written By
Balsara Jay
- Last Modified 13-01-2025
Principle of Calorimetry: Meaning, Types, Solved Examples
Principle of Calorimetry: When we talk about energy, we generally talk about the kinetic energy and potential energy of an object as a whole. Kinetic energy is the energy associated with the body’s motion, whereas potential energy is the energy associated with the position of the body. But looking at the molecular level of any gas, we have molecules separated by a certain distance. When we heat that gas, the temperature of the gas increases and the particles gets energized. Due to this energy, particles obtain kinetic energy, and they start performing random motion and the energy of the gas increases. This energy is known as the internal energy of the gas. For an ideal gas, we never consider potential energy between two particles in the case of internal energy. So, internal energy only comprises the kinetic energy of the molecules in the gas.
Now, due to this motion, heat energy is formed. Heat flows from an object with a higher temperature to a lower temperature. Hence, there is a heat current between two conducting bodies with a temperature difference or in a chemical reaction. In this article, we will learn in detail about the measurement of that heat energy.
Calorimetry and Thermometry
Heat is a form of energy that flows from higher temperatures to lower temperatures. This means we can say that temperature is a kind of medium through which heat is transferred. Without any temperature difference between two objects, there is no transfer of heat.
The branch dealing with the measurement of temperature is known as thermometry, and the device which is used to measure temperature is known as a thermometer. Whereas the branch dealing with the temperature of measurement of heat released or absorbed in any substance is known as calorimetry, and the device which is used to measure heat is known as a calorimeter.
Thus, the principle of calorimetry deals with the transfer of heat from one substance to another substance. It states that the amount of heat energy lost by any substance is equal to the amount of heat energy absorbed by another substance at a lower temperature for an insulated system. This is the ideal case for an insulated system only. In real life scenario, there is always some amount of heat lost during the heat transfer process.
Calorimeter
A calorimeter is a device used for calorimetry, or in other words, a calorimeter is a device used to measure heat during a chemical reaction, transfer of heat, or during the state of matter of any substance.
There are two basic types of calorimeters:
(1) Coffee cup calorimeter
(2) Bomb calorimeter
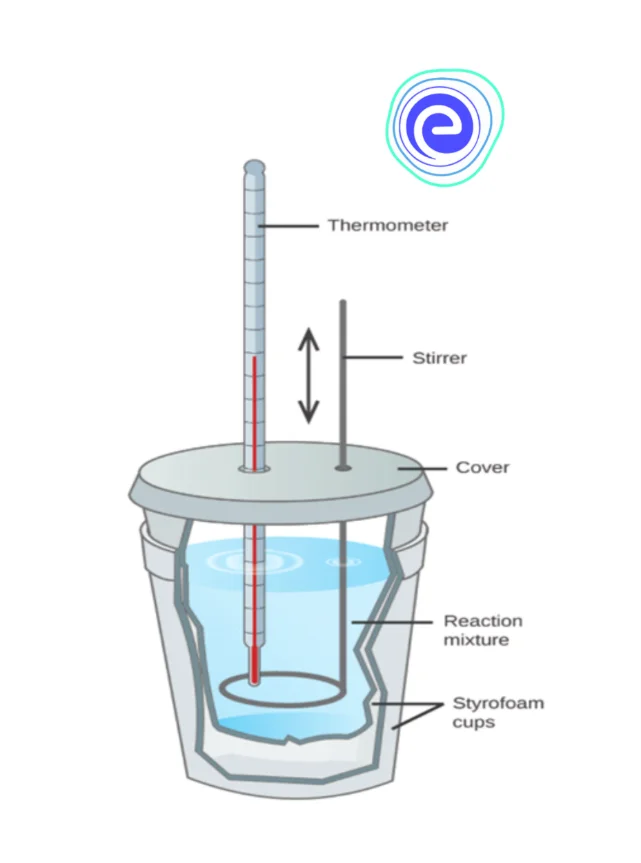
1. Coffee Cup Calorimeter
A coffee cup calorimeter is a constant-pressure calorimeter. It consists of a Styrofoam coffee cup, a thermometer, water and a reactant, which is placed inside the cup. Whenever a reaction occurs inside the coffee cup, the complete heat from the reaction is absorbed by the reactants because the coffee cup, which is made of Styrofoam, ensures that it is insulated from the external environment. The change in water temperature is measured using the thermometer, and with the help of the change in water temperature, we measure the heat absorbed.
The coffee cup calorimeter is great for measuring the heat transfer, but its only limitation is that it cannot be used in reactions that involve gases, or it can’t even be used at a higher temperature because it would melt the cup.
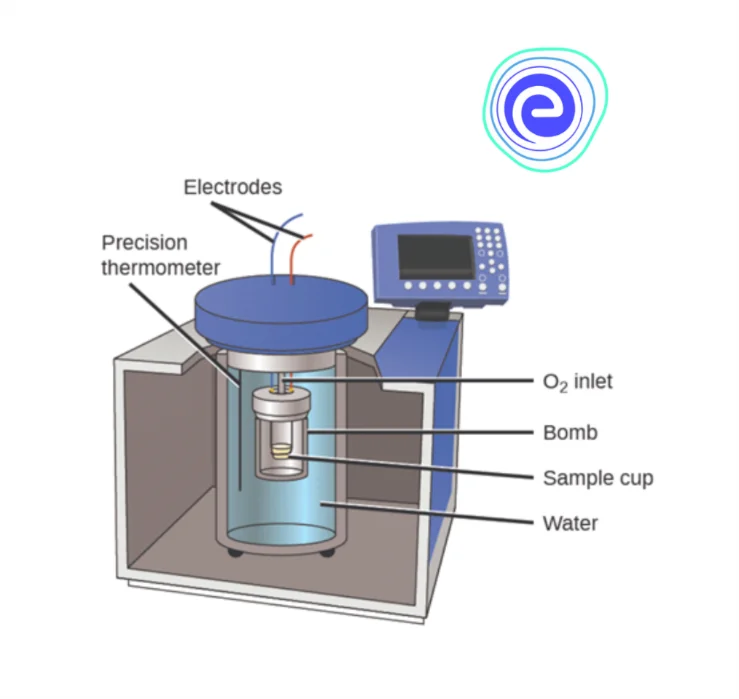
2. Bomb Calorimeter
The working of a bomb calorimeter is similar to a coffee cup calorimeter. The difference is that instead of having constant pressure, this one has constant pressure and is used to measure heat transfer involving gases.
The reaction takes place inside the sample cup, shown in the diagram, and the surrounding is covered by water. The cup layer is not insulated. That is, heat can flow from gas in the sample cup to the water.
Due to this flow of heat, the temperature of water changes. This temperature is measured using the precision thermometer, and with the help of the temperature change, the amount of heat transferred is measured.
Specific Heat and Specific Heat Capacity
Specific heat is the heat required to increase the temperature of any substance. The equation of specific heat is given by:
\({\text{Q}} = \,{\text{mCVT}}\)
Here,
\({\text{Q}}\) is the heat supplied
\({\text{m}}\) is the mass of substance whose temperature is increased
\({\text{C}}\) is the specific heat capacity of the substance
\({\text{\Delta T}}\) is the change in temperature
In the above-given calorimeters, if the thermometers observers the change in temperature, we can get the value of heat transfer. The equation of specific heat capacity is given by;
\({\text{C}} = \frac{{\text{Q}}}{{{\text{n\Delta T}}}}\)
So, if the mass is \(1\,{\text{kg}}\), the change in temperature is \(1\,{\text{K}}\) we say that \({\text{C}}\,{\text{ = }}\,{\text{Q}}\). Hence, specific heat capacity is defined as the amount of heat required to raise the temperature of \(1\,{\text{kg}}\) of a substance by \(1\,{\text{K}}\). Similarly, if we talk about gas,
\({\text{C}} = \frac{{\text{Q}}}{{{\text{n\Delta T}}}}\)
Similarly, here we define \({\text{C}}\) as molar specific heat capacity, which is the amount of heat required to raise the temperature of \(1\) mole of a substance by \(1\,{\text{K}}\)
Specific heat capacity of ice \({{\text{C}}_{{\text{ice}}}}\, = \,2.1\,{\text{kj}}\,{\text{k}}{{\text{g}}^{{\text{ – 1}}}}\,{{\text{k}}^{{\text{ – 1}}}}\)
Specific heat capacity of water \({{\text{C}}_{{\text{water}}}}\, = \,4.2\,{\text{kj}}\,{\text{k}}{{\text{g}}^{{\text{ – 1}}}}\,{{\text{k}}^{{\text{ – 1}}}}\)
Latent Heat
Latent heat is the heat required to change the state of matter of any substance at a constant temperature. The equation of latent heat is given by,
\({\text{Q}} = {\text{mL}}\)
Here, \({\text{Q}}\) is the heat supplied
\({\text{m}}\) is the mass of a substance
\({\text{L}}\) is the specific latent heat of a substance
If we take the mass of the substance to be \(1{\text{kg}}\), we will get \({\text{Q}}\,{\text{ = }}\,{\text{L}}\). Hence, specific latent heat will be defined as the amount of heat required to change the state of matter of \(1\,{\text{kg}}\) of a substance at a constant temperature.
To convert a solid into a liquid, we call it latent heat of fusion, whereas to convert liquid to gas, we call it latent heat of vaporization.
Latent heat of fusion for water is \({{\text{L}}_{\text{f}}} = \,333\,{\text{kj}}\,{\text{k}}{{\text{g}}^{ – 1}}\)
Latent heat of vaporization for water is \({{\text{L}}_{\text{v}}} = \,2256\,{\text{kj}}\,\,{\text{k}}{{\text{g}}^{ – 1}}\)
Solved Examples on Principle of Calorimetry
- \(100\,{\text{g}}\) of a sample of ice is initially at \( – 10\,\,{\text{C}}\). Determine the amount of heat required to convert it into steam completely.
Sol. First, we will raise the temperature of this sample of ice to its melting point. Then, at that temperature, ice will get converted to water. Further, the temperature of the water will be increased to its boiling point, and finally, at that temperature, it will be converted to heat.
\({{\text{Q}}1}\, = \,100 \times {10^{ – 3}} \times 2.1 \times 10 = 2.1\,{\text{kj}}\,{{\text{Q}}_2}\, = 100 \times {10^{ – 3}} \times 333\, = 33.3\,{\text{kj}}\,{{\text{Q}}{\text{3}}} = \,100 \times {10^{ – 3}} \times 4.2 \times 100 = 42\,{\text{kj}}\,{{\text{Q}}_4}\, = \,100 \times {10^{ – 3}} \times 2256\, = 225.6\,{\text{kj}}\\)
Hence, the total amount of heat required will be:
\({\text{Q}}\, = {{\text{Q}}_1}\, + \,{{\text{Q}}_2}\, + \,{{\text{Q}}_3}\, + {{\text{Q}}_4}\, = \,2.1 + 33.3 + 42 + 225.6\, = 303\,{\text{kj}}\)
2. \(500\,{\text{g}}\) of a sample of ice at \( – 20\,{\text{C}}\) is supplied with a heat of \(100\,{\text{kj}}\). What is the mass of ice that is melted and the mass of ice remaining?
Sol. The heat required to increase the temperature will be:
\({{\text{Q}}_1} = \,500 \times {10^{ – 3}} \times 2.1 \times 20 = 21\,{\text{kj}}\)
Heat remaining:
\({{\text{Q}}_{{\text{remaining}}}} = \,100 – 21 = 179\,{\text{kj}}\)
Mass of ice melted with this much amount of heat:
\({{\text{m}}_{{\text{melted}}}}\, = \frac{{79}}{{333}}\, = 237.23\,{\text{g}}\)
Mass of ice remaining:
\({{\text{m}}_{{\text{remaining}}}}\, = \,500 – 237.23\, = 262.77\,{\text{g}}\)
Summary of Principle of Calorimetry
Reading this article, we came to know in detail about calorimetry. It measures heat energy released or absorbed during heat transfer from one substance to another or during any chemical reaction. We learned about the two types of calorimeters, viz. \(\left( 1 \right)\) Coffee cup calorimeter and \(\left( 2 \right)\) Bomb calorimeter. Finally, we went into the concept of types of heat that are specific heat which is the heat required to change the temperature of a substance without changing its state of matter and latent heat, which is the heat required to change the state of matter of a substance at a constant temperature.
Specific heat: \({\text{Q}}\,{\text{ = }}\,{\text{mC\Delta T}}\)
Latent heat: \({\text{Q}}\,{\text{ = }}\,{\text{mL}}\)
Frequently Asked Questions (FAQs) on Principles of Calorimetry
Q.1. What is the law of calorimetry?
Ans: The law of calorimetry states that the heat absorbed by the cold object must be equal to the heat released by the hot object.
Q.2. What equation is used in calorimetry?
Ans: The equation involved in calorimetry is \({\text{Q}}\,{\text{ = }}\,{\text{mC}}\Delta {\text{T}}\) which is the equation for specific heat that is required to change the temperature of a substance.
Q.3. What are the types of calorimeters?
Ans: The two most important types of calorimeters are \(\left( 1 \right)\) coffee cup calorimeter and \(\left( 2 \right)\) bomb calorimeter.
Q.4. Why is calorimetry important?
Ans: Calorimetry is the measurement of heat absorbed or released during the temperature change of any substance or any chemical reaction. Since our everyday lives deal with heat transfer directly or indirectly, it is very important to know the amount of heat transferred. Hence, calorimetry is important.
Q.5. What is the most important part of a calorimeter?
Ans: The most important part of a calorimeter is the water and the reactant inside it and also the thermometer, which measures the temperature change.
We hope this article on the Principle of Calorimetry has helped you. If you have any queries, drop a comment below, and we will get back to you.