- Written By
Ankita Sahay
- Last Modified 10-01-2025
Protection of Lyophobic Sols and Gold Number
Protection of Lyophobic Sols and Gold Number: Solutions are of three types based on the particle size. They are the true solution, colloidal solution, and suspension. Colloids are homogeneous non-crystalline substances consisting of large molecules of one substance dispersed through a second substance. Some examples of colloids include gels, sols, and emulsions. Unlike suspensions, the colloidal particles do not settle and cannot be separated out by ordinary filtering or centrifuging. The dispersed phase and dispersion medium interact with each other in a colloidal solution. Based on such interactions, colloids are of two types: lyophilic and lyophobic colloids.
Lyophobic colloids are unstable in nature, and with the addition of a small amount of electrolyte, they easily get coagulated. Whereas lyophilic colloids, such as Gelatin, Starch, Gum etc., are stable towards the coagulating action of electrolytes. When a small amount of lyophilic colloid is added to a lyophobic colloid, the lyophobic colloids become more stable. This phenomenon of the action of a lyophilic colloid which prevents the coagulation of a lyophobic colloid on the addition of an electrolyte in it is known as ‘Protection’. These protective powers of different colloids are measured in terms of ‘Gold Number’. In this article, we will learn in detail about the “Protection of Lyophobic Sols and Gold Number”.
What are Lyophobic Sols?
As the name suggests, Lyophobic means ‘liquid hating’ or ‘solvent hating’. The sols which have very less or no attraction between the dispersed phase and dispersion medium (water) are called lyophobic sols. Due to less attraction, these sols are not stable and can be separated easily. In contrast to lyophobic sols, lyophilic sols have a strong force of attraction between the dispersed phase and dispersion medium.
Protection of Lyophobic Sols
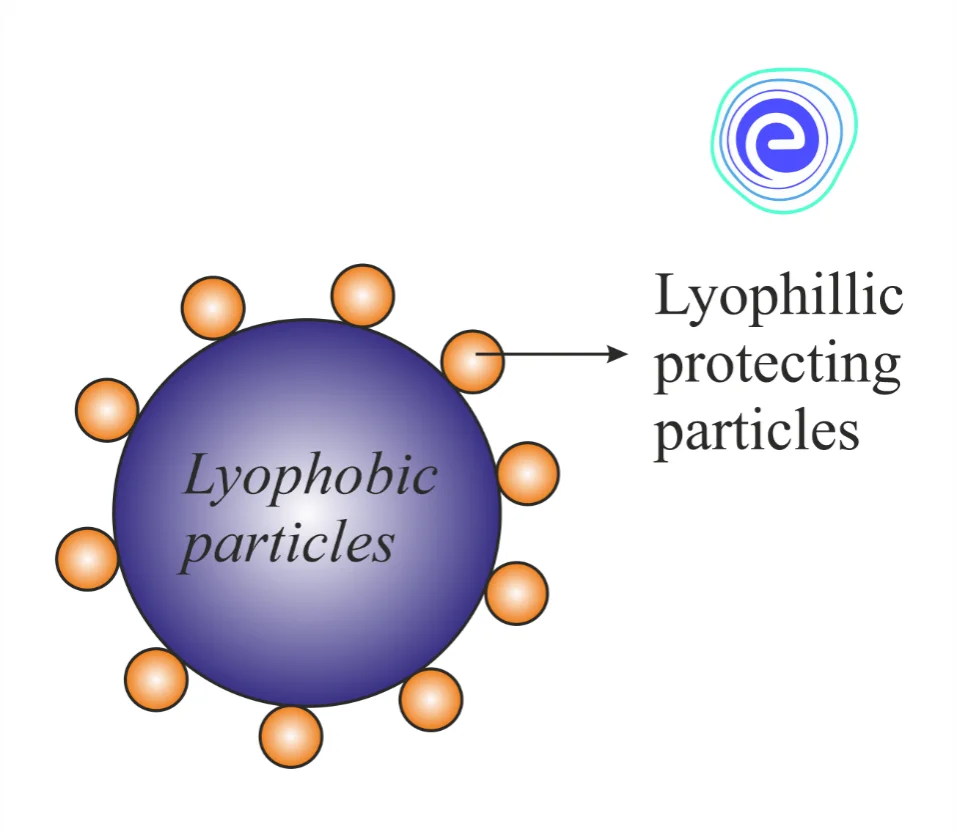
Lyophilic colloids are quite stable. Thus, if some electrolytes are added to them, no change occurs. But lyophobic colloids are unstable and can be easily get destabilized and coagulated on the addition of a small amount of electrolyte. It has been found that when a little lyophilic sol is added to a lyophobic sol, the lyophilic particles form a layer around the lyophobic particles. This layer prevents the lyophobic colloids from losing their properties by interacting with electrolytes. This kind of protective action of a lyophilic colloid that prevents the coagulation of a lyophobic colloid on the addition of an electrolyte is called ‘Protection’. The lyophilic colloid or sol added to prevent such coagulation is known as a protective or protecting colloid, and the lyophobic colloid is called a protected colloid. For example, when a small amount of Gelatin (lyophilic sol) is added to gold sol (lyophobic sol), the precipitation or coagulation of gold sol is prevented against the precipitation of \(\rm{NaCl}\) electrolyte.
Mechanism of Protection of Lyophobic Sols
The protective action of lyophilic colloids occurs due to the covering up of the particles of lyophobic sols by those of the lyophilic sols. But still, the exact mechanism of protection is not clear yet because the size of protective and protected colloidal particles is almost the same. With recent research and developments, it has been found that smaller particles get adsorbed on bigger particles. This means, if the protected particles are bigger in size than the protecting particles, the latter will be adsorbed by the former and vice versa. This would lead to the formation of a protective layer that prevents the precipitating ions from approaching the colloidal sol particles, thereby preventing coagulation or precipitation due to hindered interaction.
Gold Number
The protective powers of different lyophilic sols vary which are measured in terms of “Gold Number”. This term “Gold Number” was introduced by Zsigmondy in \(1901.\) The Gold Number is defined as the minimum weight of a protective colloid in milligrams which is required to simply prevent the coagulation of \({\rm{10}}\,{\rm{ml}}\) of a standard gold sol (i.e., gold sol containing \(0.5\) to \({\rm{0}}{\rm{.6}}\,{\rm{gm}}\) of gold per litre) by the addition of \({\rm{1}}\,{\rm{ml}}\) of \(10\% \) sodium chloride solution. Thus, it can be inferred that the lower the gold number of a protective colloid, the greater is its protective power and vice-versa. Gold number of some sols are given below as an example:
- Gold number of Gelatin is \(0.005 – 0.01.\)
- Gold number of Albumin is \(0.1 – 0.2.\)
- Gold number of Gum is \(0.15 – 0.25.\)
The above data shows that Gelatin is the best protective colloid due to its minimum gold number value.
Thus, we can say that protective power is inversely proportional to the gold number. \({\rm{Protective}}\,{\rm{Power}}\, \propto \frac{{\rm{1}}}{{{\rm{ Gold Number }}}}\)
Summary of Protection of Lyophobic Sols and Gold Number
In short, it can be inferred that the solutions are of three types based on the particle size. They are the true solution, colloidal solution, and suspension. Colloids are defined as homogeneous non-crystalline substances consisting of large molecules of one substance dispersed throughout a second substance. Gels, sols, and emulsions, etc., are some examples of colloids. In suspensions, particles are large enough to get separated through filtration or centrifugation. But on contrary, the colloidal particles do not settle down and cannot be separated out easily. Based on the interaction of dispersed phase and dispersion medium, colloids are of two types: lyophilic and lyophobic colloids.
Lyophobic means ‘liquid hating’ or ‘solvent hating’. The sols which have very less or no attraction between the dispersed phase and dispersion medium (water) are called lyophobic sols. Due to less attraction, these sols are unstable and can be separated easily. In contrast to lyophobic sols, lyophilic sols have a strong force of attraction between the dispersed phase and dispersion medium. Thus, a small amount of electrolyte easily gets coagulated. This phenomenon of the action of a lyophilic colloid which prevents the coagulation of a lyophobic colloid on the addition of an electrolyte in it is known as ‘Protection’. These protective powers of different colloids are measured in terms of ‘Gold Number’. The protective power is inversely proportional to the gold number.
Important Questions on Protection of Lyophobic Sols and Gold Number
FAQs on Protection of Lyophobic Sols and Gold Number
Q.1. Define Lyophobic sols.
Ans: Lyophobic means ‘Liquid hating’ or ‘solvent hating’. The sols which have very less or no attraction between the dispersed phase and dispersion medium (water) are called lyophobic sols. Due to less attraction, these sols are not stable and can be separated easily. In contrast to lyophobic sols, lyophilic sols have a strong force of attraction between the dispersed phase and dispersion medium.
Q.2. What is the difference between Lyophobic sols and Lyophilic sols?
Ans: Lyophobic sols are solvent repelling while Lyophilic sols have a high affinity towards the solvent. Lyophobic sols require special methods to prepare and electrolyte for their stabilisation. While, Lyophilic sols are easily prepared just by mixing, shaking, or heating substance with the dispersion medium. Lyophobic sols are unstable and can easily get coagulated by electrolytes. Lyophilic sols are highly stable and do not coagulate easily on adding electrolytes.
Q.3. What is Gold Number?
Ans: The protective powers of different lyophilic sols vary which are measured in terms of “Gold Number”. This term “Gold Number” was introduced by Zsigmondy in \(1901.\) The Gold Number is defined as the minimum weight of a protective colloid in milligrams which is required to simply prevent the coagulation of \({\rm{10}}\,{\rm{ml}}\) of a standard gold sol (i.e., gold sol containing \(0.5\) to \({\rm{0}}{\rm{.6}}\,{\rm{gm}}\) of gold per litre) by the addition of \({\rm{1}}\,{\rm{ml}}\) of \({\rm{10\% }}\) sodium chloride solution. Thus, it can be inferred that the lower the gold number of a protective colloid, the greater is its protective power and vice-versa.
Q.4. What is Sol protection or protection of colloids?
Ans: The phenomenon by which the coagulation of a lyophobic sol is prevented due to the addition of some lyophilic colloid is called sol protection or protection of colloids. The protecting power of different protective lyophilic colloids is different. The protective action of lyophilic colloids occurs due to the covering up of the particles of lyophobic sols by those of the lyophilic sols. With recent research and developments, it has been found that smaller particles get adsorbed on bigger particles. This means, if the protected particles are bigger in size than the protecting particles, the latter will be adsorbed by the former and vice versa. This would lead to the formation of a protective layer that prevents the precipitating ions to approach the colloidal sol particles thereby preventing coagulation or precipitation due to hindered interaction.
Q.5. What are the advantages of lyophilic sols?
Ans: Lyophilic sols are quite stable and do not coagulate very easily. Lyophilic sols are easily soluble or highly solvated and the particles of the dispersed phase are covered by a layer of the dispersion medium. They can protect lyophobic colloids from electrolytes.
We hope this article on the Protection of Lyophobic Sols and Gold Number has helped you. If you have any queries, drop a comment below, and we will get back to you.