- Written By
Shilpi Shikha
- Last Modified 08-01-2025
Protoplast Culture and Somatic Hybridisation: Steps, Applications
Protoplast Culture and Somatic Hybridisation: Have you heard of Pomato? It is a hybrid formed by the hybridisation of potato and tomato, as a result, the shoot of the plant bears tomato whereas the underground part bears potato. This amazing plant was the result of protoplast culture and somatic hybridisation. Protoplast is a plant cell without a cell wall.
The protoplast of two different plants is fused to produce a somatic hybrid. Somatic hybridisation involves the fusion of somatic cells to produce a hybrid. Read further to learn about protoplast culture and somatic hybridisation.
Somatic Hybridisation
Somatic Hybridisation is the process of fusing isolated protoplasts from somatic cells and growing hybrid plants from the fusion products. Somatic hybridisation allows two desirable parents’ genomes to be combined, regardless of their taxonomic relatedness. The plant of choice may not belong to the same family. It is a type of genetic manipulation in plants in which two different species of plants are fused together to create a new hybrid plant having both of its properties. Unlike sexual hybridisation or genetic engineering, somatic hybridisation offers the unique ability to mix both nuclear and cytoplasmic genes at the same time.
Learn Everything About Plant Cell Here
Steps of Somatic Hybridisation
The essential steps in the technique of somatic hybridisation are:
1. Isolation of protoplasts
2. Fusion of protoplasts
3. Culture of protoplasts to raise full plants
4. Selection of hybrid cells
5. Hybridity verification
1. Isolation of Protoplast
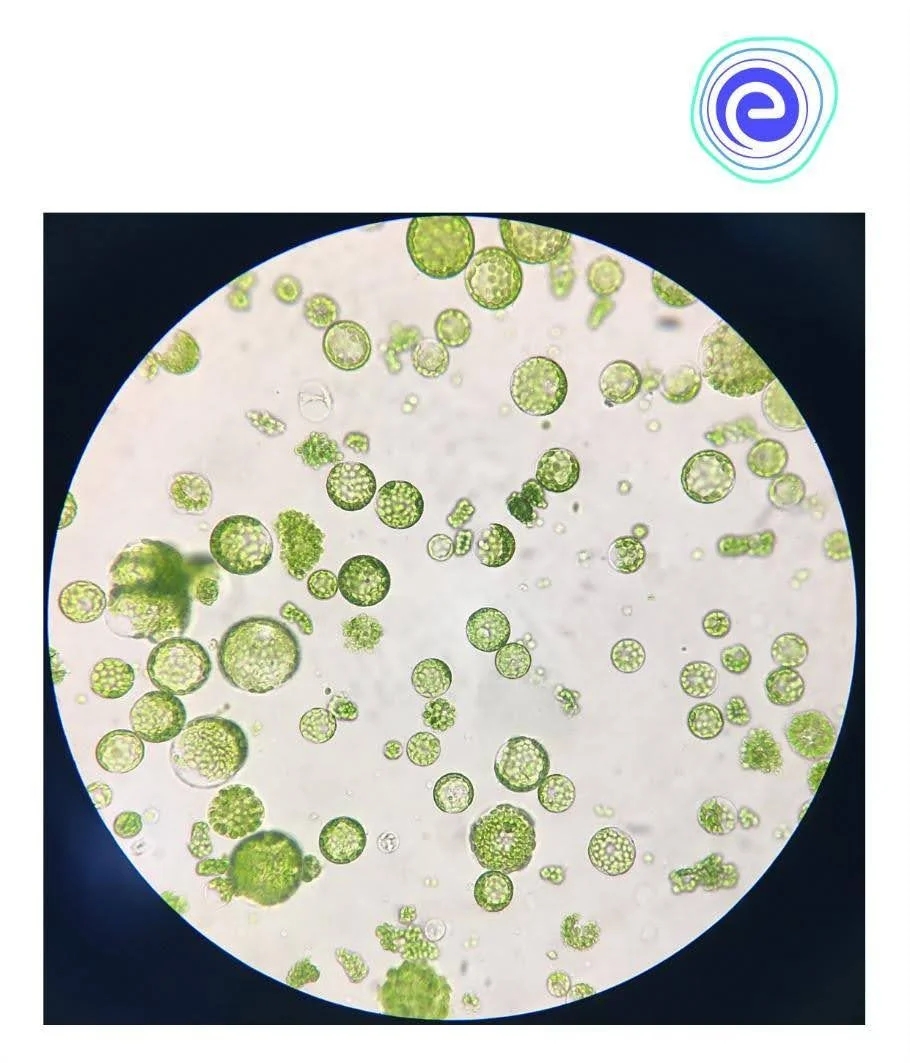
The term protoplast refers to the spherical plasmolysed content of a plant cell enclosed by plasma membranes or naked cells without a cell wall. Before diving into the mechanics of protoplast culture, it’s critical to extract viable and unharmed protoplasts as gently as swiftly as possible.
Principle
The removal of the cell wall without harming the cell or protoplasts is a crucial step in protoplast isolation. An osmotic system exists within the plant cell. The protoplasts are contained by the cell wall, which exerts an inward pressure on them. The protoplast, in turn, exerts equal and opposite pressure on the cell wall. As a result, both pressures are balanced. The equilibrium pressures will be disrupted if the cell wall is removed. As a result, the protoplast’s outward pressure will be higher, and in the absence of a cell wall, the protoplast will expand irresistibly owing to the massive intake of water from the external media. The protoplast bursts due to increased outward pressure and expansion.
Fig: Protoplast
Method of Isolation of Protoplast
Protoplast can be isolated by either of the two methods: Mechanical and enzymatic approaches.
A. Mechanical Approach: The tissue is immersed in 1.0 M sucrose until the protoplast shrinks away from their enclosing wall and then the plasmolysed tissue is cut with a sharp knife at such a thickness that only the cell walls get cut without damaging the protoplasts in strips. The protoplasts get separated by osmotic swelling when the strips of the tissue are placed in low concentration sucrose solution.
B. Enzymatic Approach: For protoplast isolation, small amounts of plant tissue, such as leaf tissue, are employed. At pH 5.4, the leaf tissue is submerged in a solution containing 0.5 per cent Macrozyme and 2% Onozuka cellulase enzymes dissolved in 13 per cent sorbitol or mannitol. It is then incubated at 25°C throughout the night.
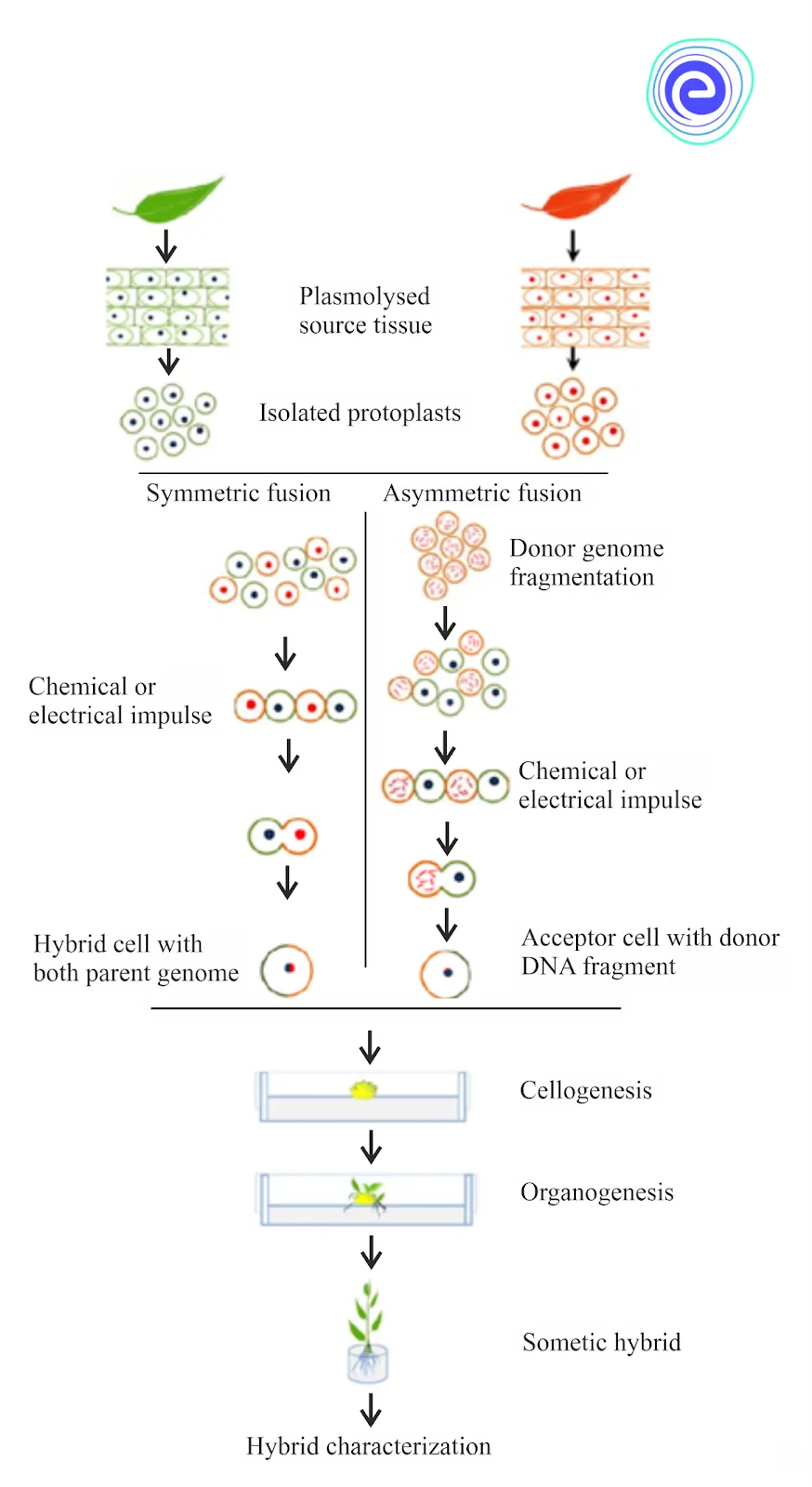
2. Protoplast Fusion
Since protoplasts lack a cell wall, they can easily fuse two genomes without encountering any incompatibility issues. Protoplast fusion can be accomplished in three ways:
A. Spontaneous fusion: During isolation, protoplasts frequently fuse spontaneously, a process known as spontaneous fusion. Protoplasts from neighbouring cells fuse through their plasmodesmata to generate multinucleate protoplasts during the enzyme treatment.
B. Induced fusion: Induced fusion is the merging ofly separated protoplasts from various origins with the assistance of fusion inducing chemical agents. Isolated protoplasts normally do not fuse with one another because their surfaces carry negative charges (-10mV to -30mV) across the exterior of the plasma membrane. As a result of their similar charges, the protoplast has a strong inclination to resist each other. As a result, fusion inducing chemicals are required, which diminish the electronegativity of isolated protoplasts and allow them to fuse with one another.
C. Chemofusion: Sodium nitrate, polyethylene glycol (PEG), and calcium ions (Ca2+) have all been used to promote protoplast fusion (also known as fusogen). Freshly separated protoplasts from the two parents are combined in the correct quantities and treated with a 15–45 percent PEG (1,500–6,000 MW) solution. The protoplasts are gradually rinsed with culture media containing a high amount of Ca2+ ions (50 mM CaCl2.2H2O) and pH adjusted to 9–10 after 15–30 minutes.
D. Electrofusion: In this approach, a low-voltage electric field (10Kv/m) causes dielectrophoretic dipole formation inside the protoplast suspension, whereas a high-voltage electric field (100Kv/m) administered for a few microseconds causes fusion.
Fig: Process of Somatic Hybridisation
3. Protoplast Culture
Protoplast culture: For the regeneration of somatic hybrid plants, protoplast culture is used. MS liquid medium is employed in droplet, plating, or micro-drop array procedures with certain modifications. Before the culture, the viability of the protoplasts is checked using fluorescein diacetate. The cultures are kept at 25°C in a constant light environment of 1000-2000 lux. The development of the cell wall takes 24-48 hours, and the first division of new cells takes 2-7 days of culture.
A cybrid is a fusion result of protoplasts lacking nuclei from separate cells. Nuclear fusion occurs as a result of this. Somatic hybridisation is the term for this process.
4. Selection of Hybrid Cells
Only around 20-25 percent of protoplasts fuse to generate a heterokaryon. Homokaryons, heterokaryons, and unfused protoplasts make up the whole combination. The selection can be done in one of three ways:
A. Biochemical approach: Biochemical substances are employed to distinguish fused cells from unfused cells in this method. There are two approaches:
a. Sensitivity to drugs: One of the protoplasts in this approach is antibiotic-resistant, and the other protoplast will be unable to develop in its presence. For example, if protoplast 1 is resistant to actinomycin D but protoplast 2 is not, the fused protoplast will take on the features of both following fusing. Proplast 2 will not be able to develop in a medium containing the antibiotic, fused protoplasts will proliferate, and protoplast 1 will form tiny colonies that can be recognised and separated.
b. Auxotrophic mutants: Auxotrophic mutants are those that are unable to develop in a minimum substrate. Because the hybrids can grow in the minimum media but the original cells cannot, the cells can be picked.
B. Visual approach: The visual approach is time-consuming since it requires visually and mechanically picking hybrid cells. Cells that grow on different mediums are fused in this manner, which allows them to be visibly separated after fusion. Another method is to manually separate the hybrid cells using a pipette called a Drummond pipette.
C. Cytometric Approach: The cytometric approach employs modern techniques such as flow cytometry and fluorescent cells to facilitate cell selection.
5. Hybridity Verification
Molecular evidence is needed to identify hybrid plants after they have developed from hybrid cells. The following are some typical methods for identifying hybrid plants.
A. Hybrid plant morphology: Hybrid plant morphology is generally a mix of the two plants’ morphologies and may be clearly distinguished. Pomatoes and topatoes are hybrids of tomatoes and potatoes that are clearly distinguishable from the mother plant.
Fig: Potato + Tomato – Pomato
B. Hybrid plant isoenzyme analysis: Isoenzymes are various versions of the same enzyme that catalyse distinct processes. Isoenzymes from one or both parents make up the hybrid plant. To confirm hybridity, these isoenzymes can be electrophoretically analysed.
C. Symmetric and asymmetric hybrids: Symmetric hybrids have the same chromosomal counts as their parents and are sterile in nature. Asymmetric hybrids, on the other hand, are abnormal because they lack a normal chromosomal number or ploidy.
Applications of Somatic Hybridisation
The applications of Somatic Hybridisation are as follows:
Disease resistance: Somatic hybridisation has allowed disease resistance genes from one plant to be spread to many others. Tomato hybrids that are resistant to illnesses like TMV and spotted wilt virus, for example, have been developed.
Biofortification: Many plants have been hybridised with desirable characteristics such as high protein content.
Environmental tolerance: Plants have been hybridised to withstand harsh environmental conditions such as cold, heat, and salt.
Cytoplasmic male sterility: The cytoplasm is in charge of a lot of things in the cell. Somatic hybridisation provides the benefit of adding characteristics such as antibiotic resistance, herbicide resistance, and male sterility into hybrids.
Somatic Hybridisation is the process of fusing isolated protoplasts from somatic cells and growing hybrid plants. Somatic hybridisation allows two attractive parents’ genomes to be combined, regardless of their taxonomic connection. Protoplast can be isolated by either of the two methods: Mechanical and enzymatic approaches. Isolated protoplasts normally do not fuse with one another because their surfaces carry negative charges (-10mV to -30mV). As a result, fusion inducing chemicals are required to allow them to fuse.
Protoplast culture is used for the regeneration of somatic hybrid plants. Auxotrophic mutants are those that are unable to develop in a minimum substrate. Another method is to manually separate the hybrid cells using a pipette and fluorescent cells. Hybrid plant isoenzymes are various versions of the same enzyme that catalyse distinct processes. Isoenzymes from one or both parents make up the hybrid plant. Symmetric and asymmetric hybrids have the same chromosomal counts as their parents and are sterile in nature.
FAQs on Protoplast Culture and Somatic Hybridisation
Q.1. Who was the first person to isolate protoplast?
Ans: Klercker was the first person to isolate protoplast successfully.
Q.2. Who was the first person to produce somatic hybrids?
Ans: Carlson et al was the first person to produce somatic hybrids.
Q.3. What was the first animal cloned?
Ans: Dolly the Sheep was the first animal cloned.
Q.4. Which is the first plant whose somatic hybrid was prepared?
Ans: Nicotiana glauca was the first plant whose somatic hybrid was prepared.
Q.5. Which was the first mammal to be cloned from a somatic cell?
Ans: Dolly the Sheep was the first mammal to be cloned from a somatic cell.
Learn About Structure of Cell Here
We hope this detailed article on the Protoplast Culture and Somatic Hybridisation helps you. If you have any queries, feel to ask in the comment section below and we will get back to you at the earliest.