- Written By
Sushmita Rout
- Last Modified 09-01-2025
Redox Reactions and Electrode Processes: Overview, Electrode Potential
Redox Reactions and Electrode Processes: We come across many things in our daily life that involve electrochemical reactions directly or indirectly. From mobile phones, flashlights to remotes – all involve electrochemical reactions and use the principle of an electrochemical cell. Electrochemical reactions are oxidation-reduction reactions in which the energy released by a spontaneous reaction is converted to electricity or electrical energy is used to initiate a nonspontaneous reaction. Let us understand how these redox reactions and electrode processes help us in carrying out our day to day activities.
Galvanic Cell/Voltaic Cell
Galvanic cell, also known as a Voltaic cell, is an electrochemical cell that uses redox reaction or redox process to produce electrical energy.
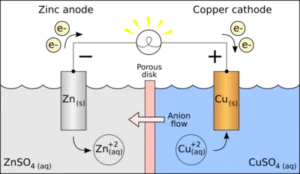
A voltaic cell consists of two half-cells where either the reduction process or the oxidation process takes place. The two half-cells are the left and right half-cells. The right half-cell consists of a copper metal rod/strip dipped in copper (II) sulfate solution, and the left half-cell consists of a zinc metal rod/strip dipped in a zinc sulfate solution. The metal strips are known as electrodes that act as a conductor in the circuit arrangement. It helps in the transfer of electrons to the electrolyte solutions in which the strips are dipped.
A metal wire connects the two electrodes in the diagram above. A switch helps to open and close the circuit. The porous membrane that connects the two halves (half-cells) helps to complete the circuit arrangement. The best method to explain the electrochemical process in a voltaic cell is to use a zinc-copper cell as an example. So, let’s look at the redox reaction between zinc and copper.
Redox Process (Oxidation & Reduction Reaction) of Zinc & Copper
In a galvanic cell consisting of zinc and copper, the zinc metal strip acts as the anode where oxidation occurs, and the copper metal strip acts as the cathode where reduction occurs. The zinc rod/strip is oxidised to \({\rm{Z}}{{\rm{n}}^{2 + }}\) ions by the direct transfer of electrons. Similarly, copper ions are reduced to copper by accepting electrons. The two half cell reactions are as follows:
(Anode) Oxidation Half-Reaction
\({\rm{Zn(s)}} \to {\rm{Z}}{{\rm{n}}^{{\rm{2 + }}}}{\rm{ + 2}}{{\rm{e}}^{\rm{ – }}}\)
(Cathode) Reduction Half-Reaction
\({\rm{C}}{{\rm{u}}^{{\rm{2 + }}}}{\rm{ + 2}}{{\rm{e}}^{\rm{ – }}} \to {\rm{Cu(s)}}\)
Zinc loses electrons that are captured by copper ions to form metallic copper. The overall zinc-copper redox reaction is:
\({\rm{C}}{{\rm{u}}^{{\rm{2 + }}}}{\rm{ + Zn(s)}} \to {\rm{Cu(s) + Z}}{{\rm{n}}^{{\rm{2 + }}}}\)
Observation of the Redox Process in Zinc and Copper Cell
In a galvanic cell, the Zinc electrode acts as the anode because zinc ranks higher in the activity series in comparison to copper and is oxidised more easily than copper.
\({\rm{Zn(s)}} \to {\rm{Z}}{{\rm{n}}^{{\rm{2 + }}}}{\rm{ + 2}}{{\rm{e}}^{\rm{ – }}}\)
The zinc anode slowly depreciates due to the loss of zinc metal, while the concentration of zinc ion gradually increases due to electron generation at the anode.
The electron moves from zinc anode through the external wire to the copper electrode, where it combines with copper ions present in the solution to form the metallic copper.
\({\rm{C}}{{\rm{u}}^{{\rm{2 + }}}}{\rm{ + 2}}{{\rm{e}}^{\rm{ – }}} \to {\rm{Cu(s)}}\)
The reduction takes place in the copper electrode that acts as the cathode. The mass of the cathode will increase due to the formation of copper metal, while the concentration of the copper (II) ions will decrease.
The passage of ions across the membrane assists in maintaining the cell’s neutrality.
Daniel Cell
A Daniel cell is the same as an electrochemical/voltaic cell. It consists of a zinc rod dipped in a beaker of zinc sulfate solution and a copper rod dipped in a beaker of copper sulfate solution. Both the solutions are connected through a salt bridge. Oxidation and reduction will occur in two half-cells of zinc and copper to form a redox couple. In Daniel cell, the redox couple is \({\rm{Z}}{{\rm{n}}^{2 + }}/{\rm{Zn}}\) and \({\rm{C}}{{\rm{u}}^{2 + }}/{\rm{Cu}}.\)
The substance undergoing oxidation and reduction is present in a redox couple. A vertical line acts as a separator or interface between the oxidised and reduced forms in a redox couple. The interface can be solid or in the form of a solution.
A salt bridge is just a \({\rm{U \,- }}\) shaped tube filled with ammonium nitrate or potassium chloride solution. The solution is solidified by boiling it with agar-agar and cooling it until a jelly-like consistency.
The salt bridge establishes the electric contact between the solutions. At the same time, it aids in the separation of the solution. A metallic wire, an ammeter, and a switch connect the copper and zinc rods. This complete set-up is an example of Daniel Cell. When the switch is turned off, there is no reaction and no current flow. However, we could observe the following things as soon as the switch is turned on.
Observations of Daniel Cell
The electronic transfer takes place over a metallic wire that connects the rods. The direction of the arrow indicates the flow of current. The movement of ions from one solution of the beaker to the other via the salt bridge causes the flow of electricity. However, current flow is not feasible until a potential difference exists between the two electrodes (copper and zinc rods).
Half-reactions are known to occur at the electrodes. As a result, the anode is the electrode where oxidation occurs, while the cathode is the electrode where reduction occurs. Electrode Potential refers to the overall potential occurring at each electrode.
Electrode Potential
The concept of electrode potential is essential to electrochemistry. It aids in predicting and controlling the direction and intensity of an electrochemical process (for example, corrosion).
Standard Electrode Potential
The electrode potential is the potential difference that occurs between the metal and its solution. The potential of the electrode is referred to as Standard Electrode Potential if the concentration of the participating species in the electrode reaction is unity and the reaction happens at \(298\;{\rm{K}}\left( {{{\rm{E}}^0}} \right).\)
Considering the case of the convention, the standard electrode potential \(\left( {{{\rm{E}}^0}} \right)\) of hydrogen gas is \(0.00\) volts.When the standard electrode potential is negative, it means the redox couple is a stronger reducing agent than the \({{\rm{H}}^ + }/{{\rm{H}}_2}\) couple. A positive standard electrode potential, on the other hand, indicates that the redox couple is a weaker reducing agent than the \({{\rm{H}}^ + }/{{\rm{H}}_2}\) couple.
Calculation of Electrode Potential
The electrode potential can be determined by:
\({\rm{E}}_{{\rm{cell}}}^{\rm{^\circ }}{\rm{ = E}}_{{\rm{red}}}^{\rm{^\circ }}{\rm{ – E}}_{{\rm{oxid}}}^{\rm{^\circ }}\)
The half-cell having the higher reduction potential undergoes reduction, while the half-cell having lower reduction potential will undergo oxidation.
Uses of Electrode Potential
- It aids in the investigation of processes such as corrosion and pitting, as well as the control of the reaction process.
- Electrode Potential is useful for selecting materials and equipment that aid in the reaction control process.
- Assists in the prediction of corrosion resulting from electrochemical and chemical reactions and processes.
Summary of Redox Reactions and Electrode Processes
Batteries are the most used items that involve electrode processes. In an electrochemical cell, electrons flow from the negative electrode to the positive electrode. Oxidation takes place in the anode, whereas reduction occurs at the cathode. In this article, we discussed the different types of electrochemical cells, such as the Galvanic cell and Daniel cell, along with the redox process that occurs at each of the electrodes in these cells. We also learnt the concept of electrode potential, its relation with the standard electrode potential and its uses.
Important Questions on Redox Reactions and Electrode Processes
FAQs on Redox Reactions and Electrode Processes
Q.1. What processes are involved in redox reactions?
Ans: A redox process denotes a coupled reduction and oxidation reaction, i.e., an electron transfer reaction where reduction is the uptake of electrons and oxidation is the release of electrons.What processes are involved in redox reactions?
Q.2. How does a redox reaction produce electricity?
Ans: Galvanic cell, also known as a Voltaic cell, is an electrochemical cell that uses redox reaction or redox process to produce electrical energy. A voltaic cell consists of two half-cells where either the reduction process or the oxidation process takes place.
Q.3. How Electrolysis is an example of a redox reaction?
Ans: Electrolysis is an example of a redox reaction because reduction takes place at the cathode and oxidation takes place at the anode, and both of these reactions take place simultaneously.
Q.4. What does the reduction process do?
Ans: During the reduction process, chemical species loses electrons and thereby decreases its oxidation number. The other part of the reaction entails the loss of electrons by oxidation. Reduction is the reverse of the oxidation process.
\({\rm{C}}{{\rm{u}}^{{\rm{2 + }}}}{\rm{ + 2}}{{\rm{e}}^{\rm{ – }}} \to {\rm{Cu(s)}}\)
Q.5. What is a redox couple example?
Ans: The oxidised and reduced forms of each reactant are referred to as a redox couple in redox processes. “Ox/red” is how redox couples are written. The oxidised version of the couple is on the left, and the reduced form is on the right, separated by a slash—for example, \({\rm{C}}{{\rm{u}}^{2 + }}/{\rm{Cu}}\) and \({\rm{Z}}{{\rm{n}}^{2 + }}/{\rm{Zn}}\)
Learn About Redox Reaction Here
We hope this article on the Redox Reactions and Electrode Processes has helped you. If you have any queries, drop a comment below, and we will get back to you.