- Written By
Nithya Samanta
- Last Modified 13-04-2025
Refining or Purification of Impure Metals
Refining or Purification of Impure Metals: Metals like Copper, Aluminium, Iron, etc., occur in nature in the combined state, in the form of their oxides, carbonates, sulphides, and so on. These are then extracted from their ‘ores’ through various extraction processes employed, depending upon the type of ore and the combination it occurs in nature. The processes employed are selected based upon their chemical feasibility and economic and commercial viability. Many metallurgical principles are used to extract these metals from their ores.
Metals, when extracted from their ores, or prepared through various reduction processes, are not always in their pure form. They contain several impurities which need to be removed before they can be used. Refining or purification of impure metals extracted through the metallurgical processes is then used to ensure the final product or the metal extracted is in its purest form.
Refining of metals is defined as the process of removal of impurities from the extracted metal. The purification of metals after their extraction is called refining. Any metal extracted from different methods, the contaminations or impurities are something that comes with it, despite the perfect selection of the extraction process. A particular refining technique is selected depending upon the type of impurities present and their difference with the properties of the metal to be refined.
Distillation
Distillation is a refining process for metals that have a lower boiling point and are volatile in nature. In the process of distillation, the impure metal is heated in a retort, and the vapours of the metal are condensed in the receiver. Since the metal to be purified has a lower boiling point, it will begin to boil well before the other non-volatile impurities are present. The non-volatile impurities are left behind in the retort. Example: Zinc, Cadmium, Mercury, etc.
Liquation
The difference in the fusibility of the metals and the impurities present in them is used to refine metals in the liquation process. When the impure metal is melted, the impurities which have a higher melting point than the pure metal are left behind. This way, one can extract the pure metal of impurities through this process. In this process, the ingots of the impure metal are heated below its melting point, on the sloping hearth of a furnace. The pure metal melts and leaves behind the impurities on the hearth. Examples of metals refined through this process include tin, lead, mercury, and so on. The metal that is refined is separated out of the higher-melting point impurities through this method.
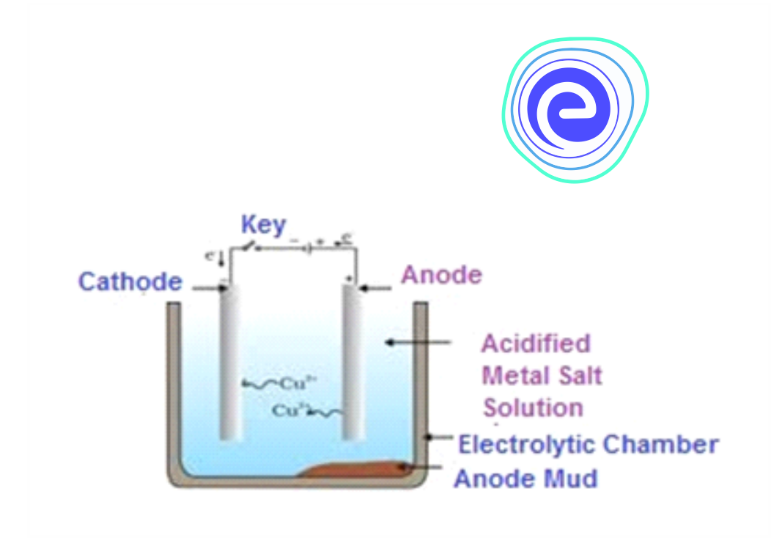
Electrolytic Refining
In electrolytic refining, the impure metal is designed as an anode, and the cathode is a strip of pure metal. An aqueous salt of the same metal is placed as an electrolyte to complete the electrolysis chamber. When an electric current is passed on, the metal ions from the electrolyte solution gets deposited at the cathode to form the pure metal. And, to replenish the metal ions lost, some amount of metal from the anode (impure metal) gets dissolved in the solution. The process continues until the pure metal leaches out from the anode completely and gets deposited in the cathode.
The impurities which are soluble passes into the solution, and the insoluble impurities settle down at the anode side as ‘anode mud’.
The reactions at anode and cathode are written as below:
\({\rm{Anode:M}} \to {{\rm{M}}^{{\rm{n + }}}}{\rm{ + n}}{{\rm{e}}^{\rm{ – }}}\)
\({\rm{Cathode:}}{{\rm{M}}^{{\rm{n + }}}}{\rm{ + n}}{{\rm{e}}^{\rm{ – }}} \to {\rm{M}}\)
Electrolytic refining is used for the purification of copper. In the electrolytic refining of copper, the impure copper is taken as an anode, and the cathode is a pure copper strip. Copper sulphate solution (acidified) is taken as an electrolyte. During electrolysis, pure copper gets deposited on the cathode from the anode. The reactions at cathode and anode are as follows:
\({\rm{Anode:Cu}} \to {\rm{C}}{{\rm{u}}^{{\rm{2 + }}}}{\rm{ + 2}}{{\rm{e}}^{\rm{ – }}}\)
\({\rm{Cathode:C}}{{\rm{u}}^{{\rm{2 + }}}}{\rm{ + 2}}{{\rm{e}}^{\rm{ – }}} \to {\rm{Cu}}\)
The anode mud, where insoluble impurities are deposited, contains antimony, selenium, silver, gold, platinum and tellurium. Electrolytic refining is used for purifying zinc metal too.
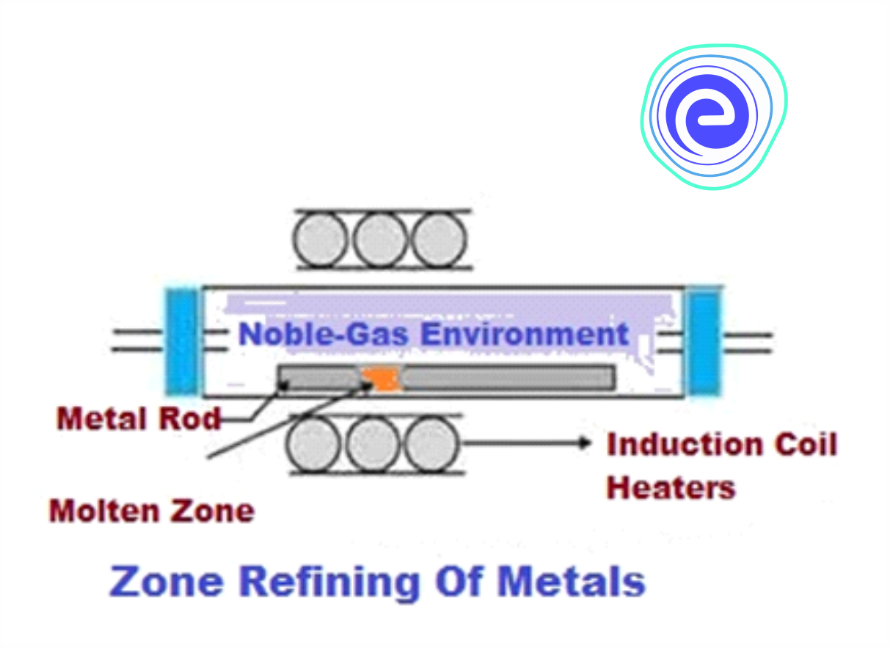
Zone Refining
The principle-based on which zone refining operates is that the impurities are more soluble in the melted phase than in the solid state of the metal. A circular mobile heater is fixed at one end of the impure metal rod. The molten zone then moves along with the heater when it is moved forward. When the heater moves in the forward direction, the pure metal crystallizes and separates out, while the molten layer containing the impurities fall down at the end of the heater. The process is repeatedly performed to get the pure metal from the impurities. The metal obtained is of high purity. This method is extremely useful in refining metals of high purity such as Si, B, In, Ge, etc. and in producing semiconductors.
Vapour Phase Refining
In vapour phase refining, the metal to be purified is changed into a volatile compound and collected separately. The non-volatile impurities are left behind. The volatile compound with the metal is then decomposed to obtain pure metal. The two mandatory factors to consider in vapour phase refining are:
- The metal to be refined should be able to form a volatile compound with the reagent used.
- The volatile compound thus formed should decompose to give metal.
Some examples of vapour phase refining are as follows:
a. Mond’s Process of refining Nickel:
Nickel is purified by Mond’s process. In this process, impure nickel metal is placed in a stream of carbon monoxide and heated to form a volatile nickel tetracarbonyl complex. This is then decomposed on heating to \({\rm{450k}}\) to yield pure nickel metal.
b. Van-Arkel Process for refining of Zirconium:
In the Van-Arkel process, the oxygen and nitrogen present in the metal as impurities are removed. The impure Zirconium metal is heated with iodine in an evacuated vessel. The metal iodide formed volatilises.
The metal iodide formed is decomposed by heating it at \({\rm{1800k}},\) using tungsten filament. The pure metal is deposited on the filament.
\({\rm{Zr (Impure) }} + 2{{\rm{I}}_2} \to {\rm{Zr}}{{\rm{I}}_4}\)
\({\rm{Zr}}{{\rm{I}}_4} \to {\rm{Zr (Pure) }} + 2{{\rm{I}}_2}\)
This process is also employed for refining or purification of Titanium metal.
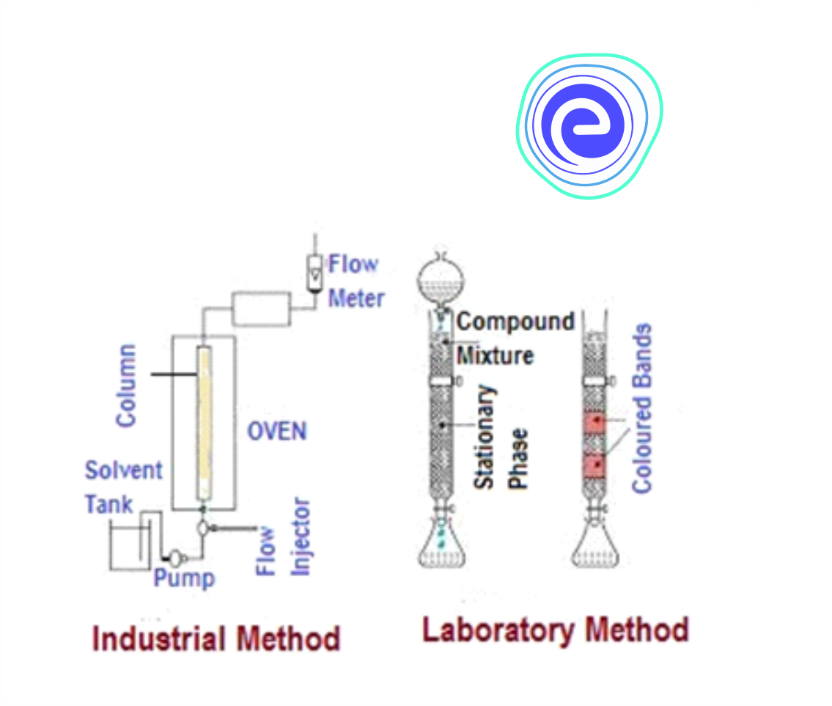
Chromatographic Methods
Chromatographic method uses the principle of selective adsorption of components in the mixture on the selected adsorbent.
Three different chromatographic techniques are used as follows:
1. Column Chromatography
2. Gas Chromatography
3. Paper Chromatography
The technique involves the use of a mobile phase and a stationary phase. The extract sample is dissolved in a mobile phase, a gas or a liquid. The stationary phase is immiscible and immobile. Different components in the mobile phase are adsorbed at different levels on column chromatography. The adsorbed components are then removed using appropriate solvents.
Metals extracted from various methods of metallurgy are not usually pure and need purification. Purification of metals is called refining, and several techniques are used to purify metals. The refining methods used for purification depends upon the impurities present in the metal and the properties of the metal. Accordingly, there are many refining techniques such as distillation, liquation, electrolytic refining, zone refining, vapour phase refining, and chromatographic methods. Each method uses a different set of principles to refine impure metal and to purify it.
Q.1. What are the methods of refining impure metals?
Ans: The methods of refining impure metals are distillation, liquation, electrolytic refining, zone refining, vapour phase refining, zone refining and chromatographic methods.
Q.2. Which method is used for the purification of copper?
Ans: Electrolytic refining is used for the purification of impure copper metal. The pure copper is taken at the cathode while the impure metal is taken as an anode, and the electrolyte used is acidified copper sulphate solution. When current is passed, the pure copper gets deposited in the cathode, while the impurities get deposited as anode mud.
Q.3. What do purification and refining mean?
Ans: purification or refining of metals is the process of removal of impurities from the impure metal extracted from its ores. The metal extracted usually contains different types of impurities and are removed using various refining techniques employed in metallurgy.
Q.4. Which metal is purified through the distillation process?
Ans: Distillation is a process of refining wherein the low boiling point metals or volatile metals are separated from higher boiling or non-volatile impurities through the distillation process. The volatile and pure metal boils out and is collected in a receiver after condensation.
Q.5. Give some examples of vapour phase refining.
Ans: Some examples of vapour phase refining include Mond’s process for refining Nickel and Van-Arkel’s process for refining Zirconium metal or Titanium metal.
Learn About Uses of Metals and Non-Metals
We hope this article on Refining or Purification of Impure Metals has helped you. If you have any queries, drop a comment below, and we will get back to you.