- Written By
Riya_I
- Last Modified 22-03-2025
Separation of Substances: Definition, Need for Separation, Different Methods
Separation of Substances: Different types of matter are familiar to us. They can exist in either a pure or impure form. There will be only one type of particle in a pure state of matter. There are no contaminants in them. Different unwanted or undesirable particles might be found in impure substances. What can be done to get rid of these particles? To get rid of them, we’ll need to use a proper separating process. How can we choose the best separating method? Why is it necessary to separate substances? How are they carried out? We will answer all these questions in this article.
Before going into separation methods, we will discuss what are pure substances and impure substances in brief.
Study About Solvent Extraction Here
Pure Substances
A pure substance is made of only one kind of particle. Therefore, a pure substance will have a definite chemical composition and distinct chemical properties. Physical separation methods cannot separate it into its constituents. Some examples of pure substances are oxygen, gold, diamond, carbon dioxide, etc.
Impure Substances
Most of the substances we find in nature are not in pure form. For example, air contains oxygen, nitrogen, carbon dioxide, and water vapour. Drinking water contains dissolved salts and air. Such substances are generally called impure substances or mixtures.
Learn 10th CBSE Exam Concepts
A mixture is made up of two or more substances mixed together in any ratio. The substances which make a mixture are called its components. These components may or may not be separated by simple physical methods. In a mixture, the components retain their individual properties. Mixtures can be of two types— heterogeneous mixture and homogeneous mixture.
Need for Separation
Why the separation of mixtures into its constituents is important? Well, it is because of the following reasons:
- Different mixtures will have different components, some of them may be useful for us. Therefore, separation helps to separate two or more but useful components.
- After making tea, we remove unwanted substance like tea leaves from the tea. Therefore, separation helps to remove the undesirable or useless components.
- Separation helps to remove the harmful components from a mixture. For example, at home, we use water purifier to remove harmful elements from the water.
- To get pure substances: When impurities are removed from a mixture, we will get pure substance from it.
Different Methods of Separation
Depending on the properties (such as particle size, colour, physical state, etc.) of the components in a mixture, different methods can be adapted to separate them. Let us discuss them in detail.
Practice 10th CBSE Exam Questions
1. Separation of Solids from Solids
If a mixture contains different solids of different properties, the following methods can be used for their separation:
(i) Handpicking:
Handpicking is used when the components of the mixture differ in their colour, size, shape and are present in small quantities. It is done using hand. For example, stones can be separated from rice with the help of handpicking.
(ii) Threshing:
Threshing is the method used to separate grain from stalks. This is done by beating the stalks against a hard surface. It can also be done with the help of bullocks or machines.
Attempt 10th CBSE Exam Mock Tests
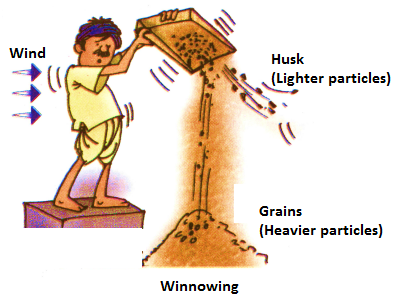
(iii) Winnowing:
It is a method of separating heavier and lighter components of a mixture with the help of wind or blowing air. This is a method generally used by farmers to separate husk from grains after threshing. The mixture of grains and husk is made to fall from a height. Grains, the heavier of the two, fall to a side, forming a large heap of their own. Husk being lighter blow’s away easily.
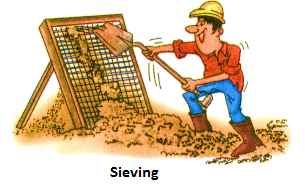
(iv) Sieving:
When the particles of a mixture differ in size, the method of sieving can be used. It is done with the help of a sieve (a kind of strainer). In construction sites, small pebbles and stones are removed from sand by the process of sieving.
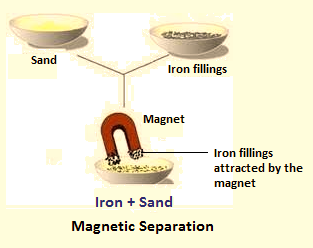
(v) Magnetic Separation:
It is done when one of the components is attracted by a magnet. For example, if we move a magnet over a mixture of sand and iron filings, iron filings will cling to the magnet and thus can be separated easily.
2. Separation of Solids from Liquids
When a solid is added to a liquid, it either dissolves or settles down in the liquid. The amount by which a solid dissolve in a liquid at a particular temperature is known as its solubility. The mixture obtained when a solid is dissolved in a liquid is called a solution.
The liquid, water can dissolve a large number of solutes. Hence, it is also called the universal solvent.
The separation of dissolved solids from a liquid can be done using the following method:
Evaporation:
This process involves the conversion of a liquid into its vapours. Therefore, when a solid is dissolved in a liquid, the liquid component of the mixture can be evaporated leaving behind the solid residue. For example, seawater contains many salts dissolved in it. To obtain salts, the seawater is allowed to stand in shallow pits. Over a period of time, water evaporates completely due to the heat of the sun, leaving behind solid salt. This is then processed and purified to obtain common salt.
The separation of an insoluble solid from a liquid can be done using the following methods:
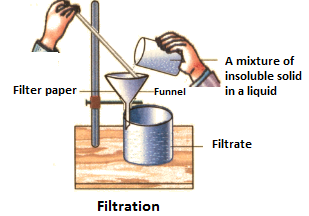
(i) Filtration:
The process of filtration is done with the help of filter paper. During the process, when a mixture containing an insoluble solid and a liquid is poured into the filter paper, the liquid will pass through it and the solid particles will remain in the filter paper. The liquid which passes through the filter paper is called filtrate and the solid left inside the filter paper is called the residue.
For example, the process of filtration can be used to separate tea leaves from the prepared tea.
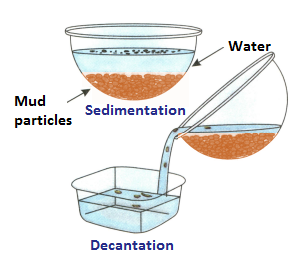
(ii) Sedimentation and Decantation:
How is mud separated from mud water? It can be done using the process of sedimentation and decantation. When muddy water is allowed to stand for a while, the heavier mud particles will settle down at the bottom. The water will remain above the mud particles. This can be poured out without disturbing the mud particles.
This process of settling down of heavier insoluble particles from a liquid is called sedimentation and the settled particles are called sediments. The liquid (here water) above the sediment is called supernatant liquid. This liquid can be poured out without disturbing the sediments. This process of pouring out the clear supernatant liquid is called decantation.
Out of filtration and decantation, filtration is the most effective method because the process of decantation is not so exact.
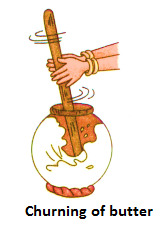
(iii) Churning (Centrifugation):
The process of separation of lighter components of a mixture from its heavier components by churning or rotating at a very high speed is called centrifugation. It is a very common method used in homes to separate butter from curd.
3. Separation of Liquids from Liquids
When two or more liquids are mixed, two types of the mixture are obtained, namely miscible and immiscible liquids.
When two liquids are miscible
When a liquid is soluble in another liquid, they are said to be miscible liquids. A mixture of alcohol and water is an example of a miscible liquid. A mixture containing miscible liquids can be separated using the process of distillation.
Distillation
It is a method to obtain pure liquid from a solution. The basic principle is similar to evaporation, but unlike evaporation, in this method, the liquid is not lost in the air but is recovered by collecting its vapours and cooling them down. The process of conversion of the vapours of a substance into its liquid state is called condensation. So, the process of distillation involves both evaporation and condensation. For example, pure water can be obtained from seawater by the process of distillation.
When two liquids are immiscible
Some liquids will not mix with each other. They are called immiscible liquids. A mixture of kerosene oil and water is an example of this. Such type of mixtures is generally separated using a specialized apparatus called separation funnel or separating funnel.
Separation Funnel:
The separation of two immiscible liquids can be done using a separation funnel. The mixture is poured into the funnel. As a result, the two distinct layers of the components will form. By decanting two layers, we can separate out two liquids.
Note: When a mixture contains more than two components, two or more methods will be necessary to separate these components. The choice of the methods depends upon the properties of the components. For example, from a mixture of sand, ink, and water, we can first separate sand by the process of filtration. As a result, we are left with a mixture of ink and water. They can be separated by distillation.
Summary of Separation of Substances
The separation of substances involves the separation of different components of a mixture. The need for separation is to obtain the desirable or pure substance from a mixture or to remove the unwanted or harmful particles from a mixture. The correct method for the separation of different components of a mixture is selected based on the properties (such as particle size, colour, physical state, etc.) of the components in a mixture. There are several methods of separation used for separating the components of a mixture. In this article, we have included them in detail.
FAQs on Separation of Substances
Q.1. Why do we separate substances?
Ans: We need to separate substances to remove unwanted or undesirable substances from a mixture.
Q.2. What are the different types of substances?
Ans: Substances are mainly classified into two types and they are pure substances and mixtures. Pure substances are either elements or compounds. A mixture is a substance that contains two or more components. They can be either homogeneous or heterogeneous.
Q.3. What is a mixture?
Ans: A mixture is a physical combination of different components. The components of a mixture are not chemically combined. Hence, they can be separated by simple processes.
Q.4. What are the benefits of separating mixtures through decantation?
Ans: The process of pouring out the clear supernatant liquid obtained after sedimentation is called decantation. It can be used to separate insoluble solids from a liquid. It can be done in a simpler way.
Q.5. What are the 5 methods of separation?
Ans: There are several methods of separation. Five of them include:
1. Sedimentation and decantation
2. Evaporation
3. Handpicking
4. Sieving
5. Filtration
Study Methods of Separation Here
We hope this article on the separation of substances is helpful to you. If you have any questions related to this post or in general about the separation of substances, please drop your comments in the comment box below and we will get back to you as soon as possible.