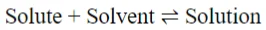- Written By
Sahana Soma Kodarkar
- Last Modified 20-01-2025
Solubility: Definition, Solubility Product, Types, Factors Affecting
Solubility: The solubility of a material refers to how well it dissolves in a solvent to produce a solution. A fluid’s solubility in another fluid (liquid or gas) might be total or partial. In general, “like dissolves like.” Solubility differences, which are represented as the distribution coefficient, are used in several separation processes (absorption, extraction). Read the article below to learn more about solubility.
Solubility
The solubility of a substance in a particular solvent at a particular temperature is the maximum quantity of the substance dissolved in a fixed quantity of the solvent to form a saturated solution at that temperature. It is determined by the nature of the solute, solvent, temperature and pressure.
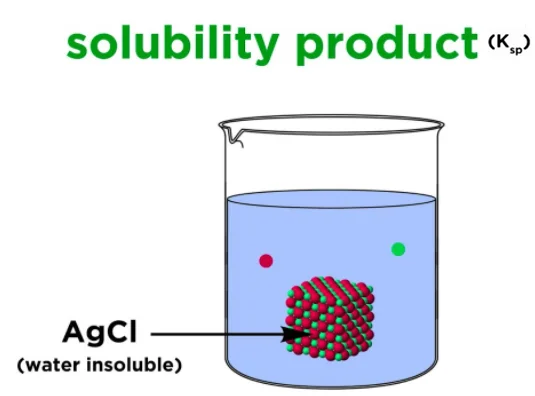
Solubility Product
The solubility product is the maximum product of the molar concentrations of the ions produced by compound dissociation. The term “solubility product” is applicable for salts that are only sparingly soluble.
The solubility product is constant at any given temperature. The lower the value of the solubility product, the lower the solubility, and the higher the value of the solubility product, the greater the solubility.
Solubility of a Solid in a Liquid
At any temperature, the solubility of a solid in a liquid is defined as the maximum amount of the solid (solute) in grams that can dissolve in \(100\,{\rm{g}}\) of the liquid (solvent) to form the saturated solution.
When a solid solute is added to a liquid solvent repeatedly, the solute dissolves and the solution’s concentration rises. A stage is achieved when no additional solute dissolves at the specified temperature which is called as dissolution. This is due to the fact that the solute particles in the solution continue to collide with the surface of solid solute particles, leading them to separate from the solution, a process known as crystallisation. No more solute dissolves because the rate of dissolution equals the rate of crystallisation, indicating that a dynamic equilibrium has been reached.
Factors affecting the solubility of a solid in a liquid
The important factors on which the solubility of a solid in a liquid depends are:
- Nature of the solute and the solvent
- Temperature
- Nature of the solute and the solvent: A solid, in general, dissolves in a chemically similar liquid. This is expressed by the phrase “Like dissolves like.” This statement implies that ionic (polar) compounds such as \({\rm{NaCl}}\) dissolve in polar solvents such as water but are very little soluble or almost insoluble in non-polar solvents such as benzene, ether, and so on. Similarly, non-polar compounds such as naphthalene and anthracene are soluble in non-polar (i.e., covalent or organic) solvents such as benzene, ether, carbon tetrachloride, and so on, but are insoluble in water.
For example,
a. Common salt (an ionic compound) dissolves in water more readily than sugar (a covalent compound). Their solubilities in water are \(5.3\) and \(3.8\) moles per litre, respectively.
b. Iodine is more soluble in alcohol or carbon tetrachloride (covalent liquids) than in water.
- Effect of temperature on solubility: The various ionic substances are classified into three groups based on the effect of temperature on water solubility.
- The solubility increases continuously with an increase of temperature. This category includes the majority of the substances such as \({\rm{NaN}}{{\rm{O}}_3},\,{\rm{KN}}{{\rm{O}}_3},\,{\rm{NaCl}},\,{\rm{KCl}}\), and others. The reason for this behaviour is that in case of all such substances, the process of dissolution is endothermic, i.e.,
\({\rm{Solute}} + {\rm{Solvent}} + {\rm{Heat}} \mathbin{\lower.3ex\hbox{$\buildrel\textstyle\leftharpoonup\over{\smash{\rightharpoondown}}$}} {\rm{Solution}}\)
- The solubility decreases with an increasing temperature. Some substances, such as cerium sulphate, lithium carbonate, sodium carbonate monohydrate \(\left( {{\rm{N}}{{\rm{a}}_2}{\rm{C}}{{\rm{O}}_3} \cdot {{\rm{H}}_2}{\rm{O}}} \right)\), and others, lose solubility as temperature rises. It is obvious that this is due to the fact that the process of dissolution of these substances is exothermic; that is, it is accompanied by heat evolution.
- The solubility of a substance does not continuously increase or decrease. Some substances, when heated, change from one polymorphic form to another or from one hydrated form to another or from hydrated to anhydrous form. Such substances do not exhibit a steady increase or decrease in solubility. For example, in the case of sodium sulphate, the solubility first increases up to \({32.4^{\rm{o}}}{\rm{C}}\) before decreasing.
Solubility of a Gas in a Liquid
The amount of gas in cc (converted to S.T.P.) that may dissolve in unit volume \(\left( {1\,{\rm{cc}}} \right)\) of the liquid to create the saturated solution at the temperature of the experiment and under one atmosphere of pressure is the solubility of any gas in a given liquid.
Almost all gases are soluble in water, though to different extents. The presence of aquatic life in rivers, seas, and other bodies of water is due to the dissolution of oxygen gas from the atmosphere in water. Some gases are also soluble in solvents such as ethyl alcohol and benzene. At a particular temperature, the solubility of a gas in a liquid is also expressed in terms of molarity (\({\rm{mol}}\) of the gas dissolved per litre of solvent to form the saturated solution, i.e., in terms of \({\rm{mol}}\,{{\rm{L}}^{{\rm{ – 1}}}}\)) or mole fraction \(({{\rm{x}}_{\rm{A}}})\) of the gas.
Factors affecting the solubility of a gas in a liquid
The important factors on which the solubility of a gas in a liquid depends on the following factors:
- Nature of gas and the solvent: Gases such as hydrogen, oxygen, nitrogen, and others dissolve only slightly in water, whereas gases such as \({\rm{C}}{{\rm{O}}_2},\,{\rm{HCl}},\,{\rm{N}}{{\rm{H}}_3}\), and others are highly soluble. The greater solubility of the latter gases is due to their reaction with the solvent. Again, at the same temperature and pressure, oxygen, nitrogen, and carbon dioxide are much more soluble in ethyl alcohol than in water, whereas \({{\rm{H}}_2}{\rm{S}}\) and \({\rm{N}}{{\rm{H}}_3}\) are more soluble in water than in ethyl alcohol. The greater solubility of a gas in a solvent is clearly due to the chemical similarity between the gas and the solvent.
- Effect of temperature: The solubility of a gas decreases as temperature rises. This is to be expected because when a gas solution is heated, some gas is usually expelled from the solution. The same result can also be obtained in a different way, as shown below:
The dissolution of a gas in a liquid is an exothermic process, i.e., it is accompanied by the evolution of heat. Thus,
\({\rm{Gas}} + {\rm{Solvent}} \to {\rm{Solution}} + {\rm{Heat}}\)
- Effect of pressure (Henry’s law): At a given temperature, this is the most important factor influencing the solubility of a gas in a liquid. A little thought reveals that the solubility increases as we compress the gas over the liquid (i.e., increase the pressure).
Solubility of Liquids in Liquid
According to the solution of liquids in one another, liquid-liquid systems are classified into the following categories.
- Completely miscible: Liquids in this system are completely miscible (soluble) when mixed in any proportion. For example, polar and polar solvents such as water-alcohol, alcohol – glycerine, water – glycerine, and so on, are said to be completely miscible because they mix in all proportions. Non-polar and non-polar solvents, such as \({\rm{CC}}{{\rm{l}}_4}\) and Benzene, are also completely miscible.
- Practically immiscible or insoluble: These liquids do not mix in any proportion. When shaken vigorously, they mix but quickly form the layers when left to stand. Chemically and polarity-wise, these liquids are entirely different to one another. For example, Castor oil (organic and non-polar) is completely immiscible with water (inorganic & polar).
- Partially miscible: The term ‘miscible’ refers to the solubility of the components in liquid-liquid systems. These liquids are miscible, but only to a limited extent, i.e. partially. These liquids mix, but they separate into two layers. Each layer is a mixture of one liquid and another. Some liquid ‘\({\rm{A}}\)’ dissolves in liquid ‘\({\rm{B}}\)’ and some liquid ‘\({\rm{B}}\)’ dissolves in liquid ‘\({\rm{A}}\)’. Both of these layers (solutions) are referred to as conjugate solutions. When such a mixture is heated, the two layers merge to form a single layer. The temperature at which two partially miscible liquids become completely miscible is known as the “critical solution consulate temperature” or “upper temperature.”
Factors Affecting the Solubility of Liquid in the Liquid
Effect of temperature: Solubility increases with increasing temperature for partially dissolving liquids such as dimethyl ether \(\left( {{\rm{C}}{{\rm{H}}_3} – {\rm{O}} – {\rm{C}}{{\rm{H}}_3}} \right)\) in water \(\left( {{{\rm{H}}_2}{\rm{O}}} \right)\), but decreases with increasing temperature for completely dissolving liquids such as ethyl alcohol \(\left( {{{\rm{C}}_2}{{\rm{H}}_5}{\rm{OH}}} \right)\) in water \(\left( {{{\rm{H}}_2}{\rm{O}}} \right)\).
Summary of Solubility
A solid, liquid, or gaseous chemical component (referred to as the solutecapacity )’s to dissolve in a solvent (typically a liquid) and create a solution is known as solubility. A substance’s solubility is mostly determined by the solvent employed, as well as temperature and pressure. The saturated solution concentration is used to determine a substance’s solubility in a given solvent. When adding more solute to a solution does not raise the concentration of the solution, it is said to be saturated.
The degree of solubility obviously varies across substances, ranging from endlessly soluble (totally miscible) ethanol in water to slightly soluble silver chloride in water. Poorly soluble chemicals are sometimes referred to as “insoluble.” The equilibrium solubility can be surpassed under certain conditions, resulting in a supersaturated solution.
The solubility of a substance in a particular solvent at a particular temperature is the maximum quantity of the substance dissolved in a fixed quantity of the solvent to form a saturated solution at that temperature. The solubility includes solubility of solid in liquid, the solubility of a gas in liquid and solubility of liquid in liquid.
Multiple Choice Questions on Solubility
- The solubility of gases increases with the decrease of
- Volume
- Mass
- Pressure
- Temperature
Ans: D
Hint: It is a physical quantity that expresses hot and cold.
- The maximum amount of solute which can dissolve 100g of solvent at room temperature is called
- Solution
- Solubility
- Tendency
- Capacity
Ans: B
Hint: It is commonly used to describe a substance in order to indicate its polarity.
- Solubility depends upon
- Temperature
- Solute
- Solvent
- All of these
Ans: D
Hint: Solubility depends on the solution and also other factors.
- The solubility of Na2SO4
- Decrease with an increase in temperature
- No effect on solubility with change in temperature
- Increases with increase in temperature
- All the above
Ans: A
Hint: As we heat the Sodium sulphate solution, sodium sulphate does not dissolve.
- Which of the following gases will be least soluble in water?
- Hydrogen chloride
- Sulphur dioxide
- Ammonia
- Nitrogen
Ans: D
Hint: It is the most abundant gas present in the air.
- Methanol and water are
- Miscible
- Immiscible
- Partially miscible
Ans: A
Hint: Methanol and water solution are homogeneous in nature.
- What happens to the solubility of gas as the pressure increases?
- Solubility decreases
- Solubility increases
- There is no effect of the pressure on the solubility
- None of the above
Ans: B
Hint: As the pressure increases, the concentration of molecules in the gas phase increases.
- For most solid solutes, what happens to the solubility as the temperature increases?
- Solubility stays same
- Solubility increases
- Solubility decreases
- Only option A
Ans: B
Hint: As the kinetic energy of a solid solution increases, the solvent can dislodge more particles from the solute’s surface.
- In a saturated solution of an electrolyte, the ionic product of their concentration is constant at constant temperature and this constant for electrolyte is known as
- Ionic product
- Solubility product
- Ionisation constant
- Dissociation constant
Ans: B
Hint: It is the maximum product of the molar concentration of the ions produced by compound dissociation.
- The mass of a gas dissolved in a given mass of a solvent at any temperature is proportional to the gas above the solvent. This statement is
- Dalton’s Law of partial pressure
- Law of mass action
- Henry’s Law
- None of these
Ans: C
Hint: This law is used in the production of carbonated beverages.
FAQs on Solubility
Q.1. How do you define solubility?
Ans: The solubility of a substance in a particular solvent at a particular temperature is the maximum quantity of the substance dissolved in a fixed quantity of the solvent to form a saturated solution at that temperature.
Q.2. What are the types of solute?
Ans: Types of solute based on the concentration of solute dissolved in a solvent that is highly soluble, sparingly soluble or insoluble.
Q.3. What are examples of solubility?
Ans: The examples of solubility are; Salt in water, sugar in water, glycerine in water, Carbon dioxide in water and hydrogen chloride gas in water.
Q.4. What \(4\) factors affect solubility?
Ans: The factors which affect the solubility are as follows:
1. Nature of solute and solvent
2. Temperature
3. Pressure
4. Polarity
Q.5. What are solubility products?
Ans: The solubility product is a type of equilibrium constant whose value varies with temperature.
\({{\rm{K}}_{{\rm{sp}}}}\) typically rises as temperature rises due to increased solubility.