- Written By
Ankita Sahay
- Last Modified 09-01-2025
Some Important Compounds of Magnesium and Calcium
Some Important Compounds of Magnesium and Calcium: Magnesium is a shiny grey solid, which is a group \(2\), or alkaline earth metal of the periodic table. Calcium is also an alkaline earth metal. It is a reactive pale-yellow metal, and when exposed to air, it forms a dark oxide-nitride layer. It is the fifth most abundant element present in the Earth’s crust and the third most abundant metal, after iron and aluminium. Although Magnesium and Calcium are in the same group, they are found in different periods of the periodic table because Calcium has one extra electron shell than that of Magnesium. Due to this reason, they have different chemical and physical properties. These metals are used in various areas, such as Magnesium is used to make aluminium alloys, die-casting (alloyed with zinc), removing sulphur in the production of iron and steel, etc.
Calcium is used in steelmaking due to its strong chemical affinity towards oxygen and sulphur. Calcium compounds are used in manufacturing insecticides, paints, blackboard chalk, textile, and fireworks. There are varieties of compounds formed by Magnesium and Calcium. Some of them are Magnesium hydroxide, Magnesium carbonate, Magnesium oxide, Magnesium chloride, Magnesium sulphate, etc. Some compounds of Calcium are Calcium hydroxide, Calcium carbonate, Calcium sulphate, etc. Now let’s learn about these compounds in detail.
Important Compounds of Magnesium
Magnesium compounds occur naturally in mineral form. Some of the compounds of Magnesium are as follows:
1. Magnesium Chloride \(\left(\mathrm{MgCl}_{2}\right)\)
Magnesium chloride is a halide of alkaline earth metal having chemical formula \(\mathrm{MgCl}_{2}\) containing one magnesium atom and two chlorine atoms. Magnesium chloride is an ionic compound as it contains magnesium \(\left(\mathrm{Mg}^{2+}\right)\) ion and chloride ions \(\left(\mathrm{Cl}^{-}\right)\).
2. Magnesium Oxide \((\mathrm{MgO})\)
Magnesium oxide, also known as ‘Magnesia’. It is an ionic compound formed by the transfer of electrons between \(\text {Mg}^{2+}\) and \(\text {O}^{2-}\). The chemical formula of Magnesium oxide is \(\text {MgO}\). It is found in nature as a white hygroscopic solid that occurs as minerals ‘periclase’. It is a mineral of magnesium that exists with metamorphic rocks in the form of the “oxides of Magnesium.” Magnesium Oxide \((\text {MgO})\) is present in the earth in the form of rocks such as Dolomite, silicate, and magnesite abundantly. Magnesium oxide is also found in the seawater and is produced mainly by the calcination of magnesite or magnesium carbonate \(\left(\mathrm{MgCO}_{3}\right)\).
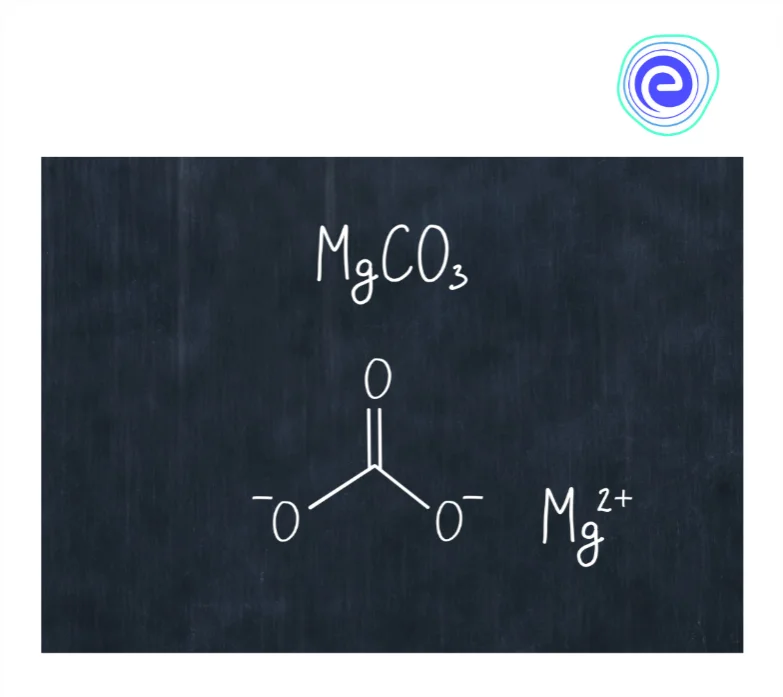
3. Magnesium Carbonate \(\left(\mathrm{MgCO}_{3}\right)\)
It is a salt made of magnesium \(\left(\mathrm{Mg}^{2+}\right)\) and carbonate \(\left(\mathrm{CO}_{3}{ }^{2-}\right)\) ions and known as magnesite. It is an ionic compound. It is formed when magnesium chloride reacts with sodium bicarbonate, magnesium carbonate \(\left(\mathrm{MgCO}_{3}\right)\), sodium chloride, water and carbon dioxide are formed. They are white solid that is hygroscopic in nature and has the power to absorb moisture from the atmosphere. Magnesium carbonate is used in making bricks, flooring, cosmetics, fire extinguishing compositions, dusting powder and toothpaste.
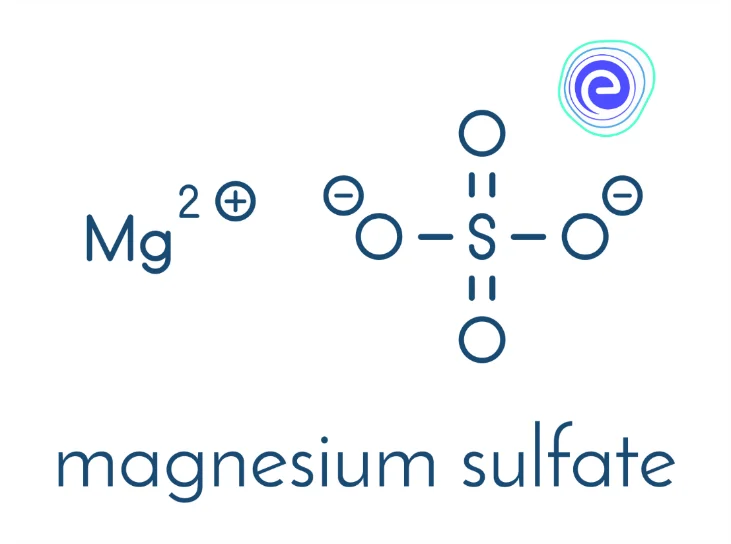
4. Magnesium Sulphate \(\left(\mathrm{MgSO}_{4}\right)\)
Magnesium sulphate is an ionic compound made up of \(\left(\mathrm{Mg}^{2+}\right)\) and sulphate \(\left(\mathrm{SO}_{4}{ }^{2-}\right)\) ions. They have a lot of medicinal values as Magnesium Sulphate is a medicine, and it is used for the treatment of low magnesium levels in the blood. It improves the blood supply to the nerve cells by widening the blood vessels in the brain.
Important Compounds of Calcium
Some of the important compounds of calcium are as follows:
1. Calcium Carbonate \(\left(\mathrm{CaCO}_{3}\right)\)
Calcium carbonate is commonly known as limestone. It occurs in nature in the form of chalk, corals, marble, calcite, etc. It is present in nature in the form of dolomite when it is mixed with magnesium carbonate. Calcium carbonate is prepared by passing carbon dioxide over slaked lime. It exists as a white solid and is insoluble in water, and liberates carbon dioxide on the action of dilute acids.
2. Calcium Oxide or Quick Lime \((\text {CaO})\)
Calcium oxide is an amorphous powder having chemical formula \(\mathrm{CaO}\) and has a melting point of \(2845 \mathrm{~K}\). Calcium oxide is prepared on a large scale from limestone by heating it in a rotary kiln at a high temperature of about \(1070\) to \(1270 \mathrm{~K}\). Carbon dioxide is rapidly removed when it is formed to help the reaction proceed in the forward direction as the reaction is reversible. On exposure to air, calcium oxide absorbs moisture and carbon dioxide forming another calcium compound slaked lime \(\mathrm{Ca}(\mathrm{OH})_{2}\) and calcium carbonate \(\mathrm{CaCO}_{3}\).
3. Calcium Hydroxide or Slaked Lime, \(\mathrm{Ca}(\mathrm{OH})_{2}\)
Calcium hydroxide can be prepared from quick lime \((\text {CaO})\) and calcium chloride. It is commonly known as Slaked lime. Calcium chloride reacts with caustic soda, calcium hydroxide is formed. A suspension of calcium hydroxide in water is known as milk of lime. This is filtered to a clear solution known as lime water.
4. Calcium Sulphate hemihydrate (Plaster of Paris)
One of the most important compounds of calcium is Calcium Sulphate hemihydrate which is popularly known as plaster of Paris. A It has chemical formula as \(\mathrm{CaSO}_{4} \cdot \frac{1}{2} \mathrm{H}_{2} \mathrm{O}\). Calcium sulphate hemihydrate is prepared by heating gypsum \(\left(\mathrm{CaSO}_{4} \cdot 2 \mathrm{H}_{2} \mathrm{O}\right)\) at \(393 \mathrm{~K}\). But this heating needs to be done in a controlled manner because, if the temperature will rise above \(393 \mathrm{~K}\), all water of crystallization will be lost, resulting in the formation of anhydrous calcium sulphate, which is also known as dead burnt plaster. Plaster of Paris is a white powder that has a remarkable settling property when comes in contact with water. This setting takes place because the plaster of Paris gets rehydrated and converts to gypsum. Plaster of Paris is used extensively to make walls, pottery, ceramics, statues, as well as in the construction industry. Plaster of Paris is used in medicine to treat fractured bones and also in dentistry.
Summary of Some Important Compounds of Magnesium and Calcium
In short, both Magnesium and Calcium belongs to the Alkaline Earth metals and are present in ‘s’ block of the periodic table. Both have two valence electrons and hence, prefers \(+2\) oxidation state, which is its stable oxidation state. Calcium forms several industrially important compounds, such as Plaster of Paris, slaked lime, Quick lime, and calcium carbonate. It is also used in dentistry for various applications involving moulds. Similarly, magnesium also forms numerous compounds such as Magnesium Chloride \(\left(\mathrm{MgCl}_{2}\right)\), Magnesium Oxide \((\mathrm{MgO})\), Magnesium Carbonate \(\left(\mathrm{MgCO}_{3}\right)\), Magnesium Sulphate \(\left(\mathrm{MgSO}_{4}\right)\).
Even biologically, Magnesium and Calcium are of utmost importance as both \(\mathrm{Mg}^{2+}\) and \(\mathrm{Ca}^{2+}\) ions are necessary for our body. Calcium is very important for bones, and Magnesium is needed for the absorption of calcium absorption as it helps in the transportation of calcium ions in and out of cells. Without magnesium, the calcium in our body will not get deposited in the right places, such as our bones. Thus, it can be inferred that the different compounds of Calcium and Magnesium are very useful in chemical laboratories, industries as well as biological purposes.
Important Questions on Some Important Compounds of Magnesium and Calcium
FAQs
Q.1. What are the important compounds of Calcium?
Ans: The important compounds of Calcium are: Calcium Carbonate \(\left(\mathrm{CaCO}_{3}\right)\), which is commonly known as limestone. It occurs in nature in the form of chalk, corals, marble, calcite, etc. Calcium carbonate is prepared by passing carbon dioxide over slaked lime. Calcium Oxide or Quick Lime is an amorphous powder having chemical formula \(\mathrm{CaO}\). Calcium Hydroxide or Slaked Lime having chemical formula \(\mathrm{Ca}(\mathrm{OH})_{2}\). It is commonly known as Slaked lime. Calcium Sulphate hemihydrate (Plaster of Paris) is one of the most important compounds of calcium is Calcium Sulphate hemihydrate is popularly known as plaster of Paris. It has chemical formula as \(\mathrm{CaSO}_{4} \cdot \frac{1}{2} \mathrm{H}_{2} \mathrm{O}\).
Q.2. What is the importance of magnesium and calcium in our bodies?
Ans: Both Calcium and Magnesium are one of the most important elements present inside our body. Calcium is very important for bones, and Magnesium is needed for the absorption of calcium absorption as it helps in the transportation of calcium ions in and out of cells. Without magnesium, the calcium in our body will not get deposited in the right places, such as our bones.
Q.3. What are the important compounds of Magnesium?
Ans: Magnesium forms several compounds such as Magnesium Chloride – Magnesium chloride is a halide of alkaline earth metal having chemical formula \(\mathrm{MgCl}_{2}\) Magnesium Oxide \((\text {MgO})\) is also an ionic compound. It is a mineral of magnesium that exists with metamorphic rocks in the form of the “oxides of Magnesium.” Magnesium Carbonate is a salt made of magnesium \(\left(\mathrm{Mg}^{2+}\right)\) and carbonate \(\left(\mathrm{CO}_{3}{ }^{2-}\right)\) ions and known as magnesite. Magnesium Sulphate \(\left(\mathrm{MgSO}_{4}\right)\) have lost medicinal values as Magnesium Sulphate as a medicine is used for the treatment of low magnesium levels in the blood. It improves the blood supply to the nerve cells.
Q.4. What is the chemical composition of Plaster of Paris?
Ans: One of the most important compounds of calcium is Calcium Sulphate hemihydrate which is popularly known as plaster of Paris. It has chemical formula as \(\mathrm{CaSO}_{4} \cdot \frac{1}{2} \mathrm{H}_{2} \mathrm{O}\). Calcium sulphate hemihydrate is prepared by heating gypsum \(\left(\mathrm{CaSO}_{4} \cdot 2 \mathrm{H}_{2} \mathrm{O}\right)\) at \(393 \mathrm{~K}\).
Q.5. Does magnesium sulphate change the pH of soil?
Ans: Solutions of magnesium sulphate are nearly neutral, thus the use of magnesium sulphate as a source of magnesium for soil does not significantly change the \(\text {pH}\) of the soil.
We hope this detailed article on Some Important Compounds of Magnesium and Calcium is helpful to you. If you have any queries, drop a comment below, and we will get back to you.