- Written By
Paramjit Singh
- Last Modified 09-02-2025
Surfactants: Definition, Mode of Action, Preparation, Types
Surfactants are a class of chemical compounds used to reduce the surface tension (or interfacial tension) between two or more compounds, such as two liquids, a gas and a liquid, or a liquid and a solid. Surfactants are amphiphilic organic molecules that are classified as organic compounds. It comprises both hydrophobic and hydrophilic groups, which is what it basically signifies.
A surfactant, in other words, has both a water-insoluble and a water-soluble component. Surfactants have the ability to diffuse in water and adsorb at air-water interfaces, which is one of their most prevalent features. It can also adsorb at the oil-water interface, where oil is combined with water. The insoluble group in water can move out of the bulk water phase and into the air or oil phase. The water-soluble head group, on the other hand, frequently remains in the water phase.
The term surfactant was coined in \(1950\) and is derived from the term surface active agent. Surfactants, in any case, are included in many of the items we use today. Detergents, wetting agents, foaming agents, emulsifiers, and dispersants all contain them. Surfactants are one of the most significant ingredients in detergents, as they aid in the removal of filth from clothing, skin, and household utensils, particularly in bathrooms and kitchens. Furthermore, they are widely used in industries. Soaps are the most common surfactants. They’re made up of lipids called triglycerides. Generally, esters are made up of trihydric alcohol, glycerol, and fatty acids with long-chain carboxylic acids. To create soaps, sodium salts of acids, and propane \(1,\,2,\,3\) triol, triglycerides are hydrolysed with a solution of sodium hydroxide. Saponification is the term for this process.
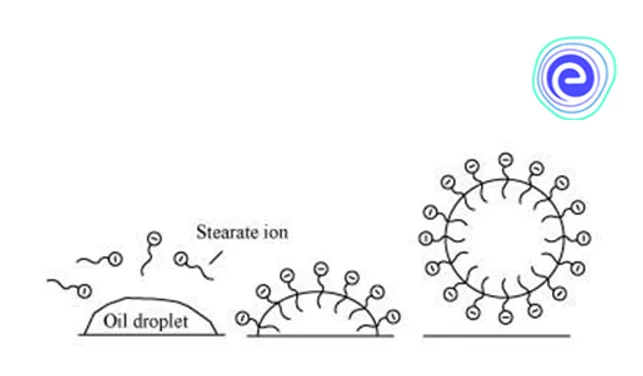
Mode of Action
When a large enough number of surfactant molecules are added to a solution, they begin to combine. In the bulk aqueous phase, they form micelles, which are structures or aggregates. The surfactant heads (hydrophilic heads) remain exposed to water or the surrounding liquid as the micelle forms. The tails (hydrophilic heads) converge at the structure’s centre, where they are shielded from water. Different types of aggregates can be generated, such as spherical or cylindrical micelles or lipid bilayers.
Furthermore, the morphology of the aggregates is largely determined by the surfactant’s chemical structure. Surfactants, in any event, function by dissolving the contact between oils, water, and dirt. The oils and grime are also suspended, making them easy to remove.
Important Points Regarding Surfactants
- Surfactants are made up of both hydrophobic and hydrophilic components.
- The surfactant molecules are absorbed by the oil, and the surfactant is therefore removed from the surface.
- Surfactant molecules wrap the oil after it has been removed, preventing it from accumulating again.
- Surfactant irritancy on the skin is thought to be due to their physicochemical qualities. Toxic and mild surfactants are the two types of surfactants that can be distinguished. Ionic surfactants will be mild, but non-ionic surfactants will be harmful.
- Surfactants are substances that reduce the physical phenomena that occur when two liquids, a liquid and a solid, or a gas and a liquid, come into contact.
Preparation of Surfactants
Unsaturated and saturated carboxylic acids with an even number of carbon atoms in the range of \(12 – 20\) are found in glycerides used to make surfactants, such as stearic acid, \({\rm{C}}{{\rm{H}}_3}{\left( {{\rm{C}}{{\rm{H}}_2}} \right)_{16}}{\rm{C}}{{\rm{O}}_2}{\rm{H}}\). Synthetic surfactants have one significant advantage over soaps. Because soaps generate insoluble magnesium and calcium salts with magnesium and calcium ions in hard water and clays found in the dirt, a lot of soap is wasted in the process of forming an insoluble mess. However, by employing a synthetic surfactant, this can be prevented. For instance, in anionic surfactants, the carboxylate group is replaced by sulfonate as the hydrophilic component.
Types of Surfactants
Surfactants are divided into numerous categories based on their polar head group. Hydrophobic tails are frequently seen to be identical. Let’s take a brief look at the information below.
Anionic Surfactants
The surfactant is called anionic if the charge on the head group (hydrophilic end) is negative. It has anionic functional groups, including sulphate, sulfonate, phosphate, and carboxylates at its head. Sulfates, sulfonates, and gluconates are examples of anionic surfactants.
Cationic Surfactants
Similarly, cationic means the head group (hydrophilic end) has a positive charge. Cationic surfactants include alkyl ammonium chlorides, which are commonly used.
Zwitterionic Surfactants
On their hydrophilic end, zwitterionic surfactants, also known as amphoteric surfactants, have both positive and negative charges. On the same molecule, they have both cationic and anionic centres. It basically has a net charge of zero. Surfactants of this kind include betaines and amino oxides.
Non-Ionic Surfactants
Non-ionic surfactants are typically neutral, and their hydrophilic end has no charge. Covalently bound oxygen-containing hydrophilic groups are bonded to hydrophobic parent structures in non-ionic surfactants. They can be used to emulsify oils and appear to remove organic soils more effectively than anionic surfactants. Non-ionic surfactants are less susceptible to water hardness and produce less foam than anionic surfactants. Ethoxylates, alkoxylates, and cocamide are examples of non-ionic surfactants.
Uses of Surfactants
Surfactants such as emulsifiers, foaming agents, and wetting agents are used depending on the application. Surfactants are at the heart of interfacial chemistry because they reduce surface tension with respect to the phase. Cleaning, wetting, dispersing, emulsifying, foaming, and anti-foaming agents all use surfactants. Herbicides (some), insecticides, biocides (sanitisers), and spermicides are among the agrochemical formulations that contain them.
Cosmetics, shampoos, shower gel, hair conditioners, and toothpaste are all examples of personal care items that contain them. Surfactants are commonly used in firefighting and pipeline construction (liquid drag-reducing agents). Oil wells also use alkali surfactant polymers to mobilise oil. Surfactants are sometimes added to car engine lubricants to help prevent particles from adhering to engine components. Surfactants are also extensively employed in ore flotation to avoid corrosion.
Summary of Surfactants
- Surfactants are a class of chemical compounds used to reduce the surface tension (or interfacial tension) between two or more compounds, such as two liquids, a gas and a liquid, or a liquid and a solid.
- Soaps are the most common surfactants, and they’re made up of lipids called triglycerides, which are generally esters made up of trihydric alcohol, glycerol, and fatty acids with long-chain carboxylic acids.
- Surfactants are made up of both hydrophobic and hydrophilic components. The surfactant molecules are absorbed by the oil, and the surfactant is therefore removed from the surface. Surfactant molecules wrap the oil after it has been removed, preventing it from accumulating again.
- Unsaturated and saturated carboxylic acids with an even number of carbon atoms in the range of \(12 – 20\) are found in glycerides used to make surfactants, such as stearic acid, \({\rm{C}}{{\rm{H}}_3}{\left( {{\rm{C}}{{\rm{H}}_2}} \right)_{16}}{\rm{C}}{{\rm{O}}_2}\)
- Surfactants are divided into numerous categories based on their polar head group. Hydrophobic tails are frequently seen to be identical.
- Surfactants such as emulsifiers, foaming agents, and wetting agents are used depending on the application. Surfactants are at the heart of interfacial chemistry because they reduce surface tension with respect to the phase. Cleaning, wetting, dispersing, emulsifying, foaming, and anti-foaming agents all use surfactants.
FAQs On Surfactants
Q.1. What are surfactants? Ans: Surfactants are a class of chemical compounds used to reduce the surface tension (or interfacial tension) between two or more compounds, such as two liquids, a gas and a liquid, or a liquid and a solid. Surfactants are amphiphilic organic molecules that are classified as organic compounds. It comprises both hydrophobic and hydrophilic groups, which is what it basically signifies. A surfactant, in other words, has both a water-insoluble and a water-soluble component. Surfactants have the ability to diffuse in water and adsorb at air-water interfaces, which is one of their most prevalent features.
Q.2. How do surfactants work? Ans: When a large number of surfactant molecules are added to a solution, they begin to combine. In the bulk aqueous phase, they form micelles, which are structures or aggregates. The surfactant heads (hydrophilic heads) remain exposed to water or the surrounding liquid as the micelle forms. The tails (hydrophilic heads) converge at the structure’s centre, where they are shielded from water. Different types of aggregates can be generated, such as spherical or cylindrical micelles or lipid bilayers. Furthermore, the morphology of the aggregates is largely determined by the surfactant’s chemical structure.
Q.3. What are anionic surfactants? Ans: The surfactant is called anionic if the charge on the head group (hydrophilic end) is negative. It has anionic functional groups, including sulphate, sulfonate, phosphate, and carboxylates at its head. Sulfates, sulfonates, and gluconates are examples of anionic surfactants.
Q.4. What are the main uses of surfactants? Ans: Surfactants such as emulsifiers, foaming agents, and wetting agents are used depending on the application. Surfactants are at the heart of interfacial chemistry because they reduce surface tension with respect to the phase. Cleaning, wetting, dispersing, emulsifying, foaming, and anti-foaming agents all use surfactants. Herbicides (some), insecticides, biocides (sanitisers), and spermicides are among the agrochemical formulations that contain them. Cosmetics, shampoos, shower gel, hair conditioners, and toothpaste are all examples of personal care items that contain them.
Learn About Applications Of Chemistry Here
We hope this article on Surfactants has helped you. If you have any queries, drop a comment below, and we will get back to you at the earliest.