- Written By
Shreya_S
- Last Modified 14-03-2025
Transport of Solutes : Diffusion, Osmosis and Facilitated Transport
Transport of Solutes: The practice of moving or distributing various resources or items from one location to another is known as transportation. Humans, in general, use a variety of modes of transportation. Similarly, the living systems, which includes plants, animals, and humans, have a complex network of transportation systems that move food, minerals, hormones, oxygen, carbon dioxide, and waste products, among other things.
All living things require the transfer of ions across membranes. As a result, it is strictly regulated and virtually always mediated by membrane-bound transport proteins. Mineral salts are absorbed and transported within plants in their ionic state by plants. However, other solutes, such as carbohydrates, are transported across the membranes uncharged. Let’s learn about the transport of solutes in plants across membranes.
Types of Solutes
The types of Solutes are as follows:
- Non Electrolyte: The transport of uncharged solutes (non-electrolytes) across the membrane is determined by their concentration gradient, which is the chemical potential gradient on only two sides of the membrane.
- Electrolyte: The situation is different in the case of charged solutes or ions (electrolytes). Because charged solutes or ions have an electric charge, their mobility across the membrane is influenced by both their chemical and electrical potentials.
Diffusion
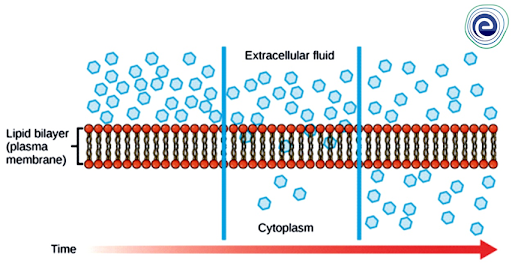
Diffusion is a type of passive transport. A single substance will tend to travel from a high-concentration area to a low-concentration area until the concentration is equal throughout the space. Passive transport does not require the cell to expend any energy hence diffusion is a type of passive transport. Molecules diffuse according to their concentration gradient; different substances in the medium will diffuse at different rates depending on their particular gradients within a system.
After the material has diffused entirely through a space, eradicating its concentration gradient, molecules will continue to move around in the space, but there will be no net movement of molecules from one area to another, resulting in a state known as dynamic equilibrium. The mass of the solute, the temperature of the environment, the solvent density, and the distance travelled are all parameters that influence the rate of diffusion of a solute.
Fig: Diffusion
Osmosis
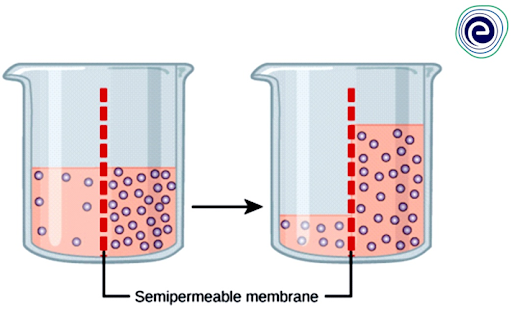
The concentration gradient of water across the membrane, which is inversely proportional to the concentration of solutes, causes osmosis to occur.Osmosis occurs when the concentration gradient of water reaches zero or when the water’s hydrostatic pressure equals the osmotic pressure. When there is a concentration gradient of a solute within a solution, but the membrane prevents the solute from diffusing, osmosis occurs.
Fig: The water can pass through the selectively permeable membrane but not the solute
Tonicity
Tonicity, which is proportional to a solution’s osmolarity, influences osmosis by influencing the direction of water flow. The total solute concentration of a solution is described by its osmolarity; low osmolarity solutions have a low solute concentration, whereas high osmolarity solutions have a high solute concentration. Water travels from the lower osmolarity (and more water) side of the membrane to the higher osmolarity (and less water) side.
The extracellular fluid in a hypotonic solution has a lower osmolarity than the fluid inside the cell, allowing water to enter the cell. The extracellular fluid in a hypertonic solution has a higher osmolarity than the fluid inside the cell, and water leaves the cell. There will be no net flow of water into or out of the cell in an isotonic solution since the extracellular fluid has the same osmolarity as the cell.
Facilitated Transport
Facilitated transport is a type of passive transport. Unlike simple diffusion where materials pass through a membrane without the help of proteins, in facilitated transport, also called facilitated diffusion, materials diffuse across the plasma membrane with the help of membrane proteins.
A concentration gradient exists that would allow these materials to diffuse into the cell without expending cellular energy. However, these materials are ions or polar molecules that are repelled by the hydrophobic parts of the cell membrane.
Facilitated transport proteins shield these materials from the repulsive force of the membrane, allowing them to diffuse into the cell. The material being transported is first attached to protein or glycoprotein receptors on the exterior surface of the plasma membrane. This allows the material that is needed by the cell to be removed from the extracellular fluid. The substances are then passed to specific integral proteins that facilitate their passage. Some of these integral proteins are collections of beta-pleated sheets that form a channel through the phospholipid bilayer. Others are carrier proteins which bind with the substance and aid its diffusion through the membrane.
Active Transport
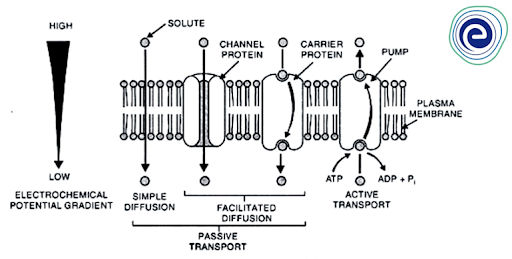
Active transport occurs when solute transfer across the membrane occurs against a chemical potential gradient or an electrochemical potential gradient, and therefore necessitates additional energy input.
Fig: Active transport
Passive Transport
If the solutes are transported across the membrane following a chemical potential gradient or an electrochemical potential gradient, it is referred to as passive transport (for non-electrolytes and electrolytes respectively).
Transporter Proteins
Influx is the transport of solutes into the cytosol across a membrane (such as the plasma membrane or the tonoplast), whereas efflux is the movement of solutes out of the cytosol. These transporter proteins work because they are extremely selective and have a complicated structure.
These membrane transporter proteins can be grouped in three categories:
(i) Ion-channels
(ii) Carriers
(iii) Pumps
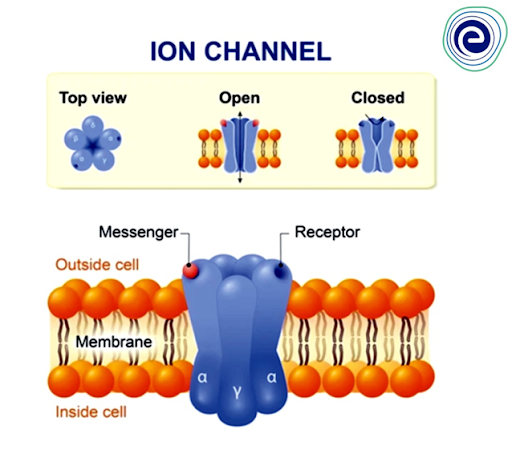
1. Ion-channels
Ion-channels are transmembrane proteins that serve as selective apertures for ions to easily diffuse across the membrane. Ion channels are often extremely selective for only one or a few ion species. More than the selective binding of ions, the specificity is determined by the size of the pore and the density of surface electric charges on its interior lining. Ions are always transported passively through channels.The channels are ‘gated,’ meaning they aren’t always open. External stimuli that open or close the gates include :
(i) Voltage fluctuations
(ii) Light
(iii) Hormone binding
(iv) Ions themselves.
Fig: Ion channels
Ions can diffuse via the channels when the gates are open, but not when they are closed. The sensing region or sensor in channel proteins is thought to respond to the proper stimulus by changing the conformation of the channel protein and opening the gate. Because the ions have a charge and are mobile, their diffusion across the membrane channel generates an electric field that can be monitored using a technique called “patch-clamp electrophysiology.”
- Patch-clamp experiments have shown that a particular membrane has a number of channels for a given ion, such as K+, that open in different voltage ranges or in response to different stimuli, such as K+ and Ca2+ concentrations, pH, protein kinases and phosphatases, and so on.
- Ions can diffuse at speeds of up to 108 s-1 through an open channel.
- Inward rectifying or inward channels allow inward ion transport (i.e., towards the cytosol side), whereas outward rectifying or outward channels allow outward diffusion (i.e., from the cytosol to another side).
- Ca2+ channels are always outward rectifying, whereas anion channels are always inward rectifying (for transport of such ions in the reverse direction, active transport mechanisms are required)
- K+ is an exception. It can diffuse inside or outward across the membrane through channels, depending on whether the membrane potential is more negative or more positive.
- Many channel proteins are inducible, meaning that the cell produces them when a certain solute is accessible for absorption.
2. Carriers
Instead of forming pores in the membrane, these trans-membrane transporter proteins selectively bind the solute to be delivered to a specific spot on them. This triggers a conformational shift in the carrier protein, allowing the solute to cross the membrane to the other side. The carrier protein reverts to its natural shape when the solute is released from the binding site in order to pick up a new solute molecule or ion. As a result, the binding and release of a solute via a carrier resemble an enzyme-catalyzed process.
Carrier-mediated solute transport transports a significantly larger range of solutes than channel-mediated solute transport, although it is slower (approximately 104–105 s-1) than channel-mediated solute transport. Carrier mediated solute transport may be of two types:
a. Passive transport
b. Active transport
Fig: Carrier Proteins
3. Pumps
Pumps are membrane transporter proteins that are engaged in the major active transport of solutes, as previously stated. Ion pumps move ions such as H+ and Ca2+ across the membrane and are the most common type of pump. Large organic solutes may be transported across the membranes by some pumps (such as those in the ABC transporters category).
Ion-pumps may be of two types:
(i) Electro neutral pumps and
(ii) Electrogenic pumps.
The term “Electron neutral pumps” refers to pumps that transport ions across a membrane with no net charge movement. Some animal cells’ H+/K+-ATPase, for example, pumps out one H+ for every K+ taken in, with no net charge movement. As a result, it is referred to as an electroneutral pump.
Electrogenic pumps, on the other hand, transport ions by moving charge across the membrane in a net manner. H+-ATPase, for example, pumps out H+ with a net positive charge movement in plant and animal cells. As a result, it’s a pump that generates electricity. (An example of an electrogenic pump is the Na+/K+ ATPase found in animal cells such as neurons, which expels three Na+ ions for every two K+ ions taken in, resulting in a net outward movement of one positive charge.) The most prevalent electrogenic pumps in plant cells are H+-ATPase, H+-PPase, and Ca2+-ATPase, all of which pump outward. (As a result, alternative mechanisms (secondary active transport) are required for the majority of mineral nutrients to be absorbed.)
A brief account of some of the most common pumps in plant cells is as follows:
(i) Proton-ATPase Pumps (H+-ATPases):
The plasma membrane, tonoplast, and potentially other cell membranes contain these pumps, which are also known as P-type ATPases. This enzyme protein has ten hydrophobic transmembrane segments or domains and is a single chain polypeptide.Hydrophilic loops projecting in the cytosol and cell wall connect these segments (apoplast). An aspartic acid residue (D) on a loop connecting the 4th and 5th segments towards the cytosolic side is thought to represent the ATP binding site. When ATP is hydrolyzed, the protein undergoes a conformational change, and one H+ ion is transferred from the cytosol to the plasma membrane. The H+ – ATPases of the plasma membrane and tonoplast are different.
(ii) Proton-pyrophosphatases (H+– PPases):
They’re mostly found in tonoplasts, although they can also be found in Golgi-body membranes. They inject protons into the vacuole and Golgi-cisternae lumens. To establish a protons gradient across the tonoplast, these pumps appear to function in tandem with vacuolar ATPases. This enzyme protein is made up of a single polypeptide chain with a molecular mass of 80 kD that gets its energy from inorganic pyrophosphate hydrolysis (PPi). The energy released by PPi hydrolysis is lower than that released by ATP hydrolysis. Only one H+ per PPi molecule hydrolyzed is transported by vacuolar H+ – PPase.
(iii) Calcium Pumping ATPases (Ca2+-ATPases):
These are found in the plasma membrane, tonoplast, and possibly other cell membranes such as chloroplast and endoplasmic reticulum membranes. These pumps combine ATP hydrolysis with Ca2+ translocation across the membrane.
(iv) ATP-Binding Cassette Transporters (ABC Transporters):
Large metabolites like anthocyanins and other secondary plant products are taken from the cytosol and transported to the vacuole by ABC-transporters on the tonoplast that consumes ATP directly. ABC transporters have recently been discovered in the plasma membrane and mitochondria.
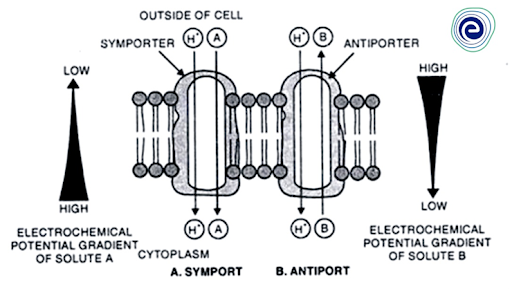
Secondary Active Transport—Symport and Antiport:
A secondary active transport mechanism that does not directly use energy liberated by ATP hydrolysis but indirectly through the energy stored in the proton-electrochemical potential gradient across the membrane or the proton motive force transports a large number of nutrients across cell membranes against their chemical potential or electrochemical potential gradients. Proton translocating carrier proteins, the electrogenic proton-ATPase (H+-ATPase) pumps store the energy of ATP hydrolysis in the form of a proton gradient across the membrane (more protons accumulating on the outer side).
This proton gradient, when combined with the usual membrane potential, results in a proton electrochemical potential gradient, or a proton motive force, which tends to transfer protons back across the membrane via specialised carrier proteins situated elsewhere on the membrane. When protons return to the cytosolic side, the proton motive force generated by electrogenic H+ transport can be used to drive the transport of other solute molecules or ions against their chemical or electrochemical potential gradient through the same carrier protein that protons are returning to the cytosol through. This is known as secondary active transport, and this type of solute transport is also known as cotransport mechanism because ions or molecules of two separate substances (H+ and other solutes) are transported at the same time by some carrier protein. Secondary active transport or cotransport mechanism is of two types:
(i) Symport and
(ii) Antiport
Fig: Symport and Antiport
Symport: The cotransport process is known as symport, and the carrier protein is known as a symporter when the inflow of protons is combined with the movement of other solutes in the same direction.
Antiport: The cotransport mechanism is called antiport, and the carrier protein involved is called an antiporter when the influx of protons is combined with the efflux of other solutes.
Summary
When moving in the direction of a rising electrochemical potential gradient, solute transport is active, and when moving in the direction of a decreasing electrochemical potential gradient, it is passive. Pumps and carriers, which have different forms of energization, are involved in active solute transport. Pumps are fueled by metabolic energy (and sometimes redox or light energy), whereas active carriers are energised by ionic gradients across the membrane. Pumps carry out primary active transport, which is virtually always fueled by ATP.
Primary active transport’s responsibility is to develop an ion gradient (usually a proton gradient in plants), which is then used to energise Secondary Active transport mediated by Carriers. The passive transport of solutes is mediated by (ion) channels or carriers, the latter of which is sometimes known as uniporters. Pumps, carriers, and channels all have varying turnover rates, with pumps being the slowest, carriers intermediate, and channels fastest. Pumps and carriers have high-affinity substrate binding sites and multiple conformational changes during the catalytic cycle, whereas channels have less stringent substrate binding and modest conformational changes. Symporters transport both substrate and driver ions in the same direction, antiporters carry substrate and driver ions in opposing directions, and uniporters transport solely substrate.
FAQs on Transport of Solutes
Q.1. Does diffusion involve the transport of solutes?
Ans: Osmosis moves only water over a membrane, whereas diffusion transports materials across membranes and within cells. The diffusion of solutes in the water is limited by the semipermeable barrier.
Q.2. What direction do solutes move during diffusion?
Ans: Diffusion is described as transporting solutes “down the concentration gradient” because it moves materials from a higher concentration area to a lower concentration area. The end outcome is an equal concentration of molecules on both sides of the membrane or equilibrium.
Q.3. What is the importance of diffusion in the transport of solutes into the cells?
Ans: Diffusion is crucial to cells because it helps them to collect the necessary components for energy and growth, as well as dispose of waste products.
Q.4. Do solutes move during osmosis?
Ans: Water travels from areas of low solute concentration to areas of high solute concentration in osmosis.
Q.5. Which three transporters can let ions into a cell?
Ans: Facilitated diffusion is enabled by three categories of transport proteins: channel proteins, gated channel proteins, and carrier proteins. A channel protein is a type of transport protein that works as a pore in the membrane, allowing tiny ions or water molecules to pass through swiftly.
Learn About Cell Wall Here
We hope this detailed article on the Transport of Solutes will help you. If you have any queries leave a comment below and we will get back to you at the earliest.