- Written By
Pavithra VG
- Last Modified 23-01-2025
Uses of Carboxylic Acids: Structure & Properties
Uses of Carboxylic Acids: What does vinegar taste like? What is its purpose? What chemical compound is it made up of? Vinegar has a sour taste and is commonly used in cooking. Vinegar is an aqueous solution of ethanoic acid (acetic acid) with a concentration of \(5-8\) percent. Acetic acid is this case.
Other carboxylic acids include formic acid, butanoic acid, benzoic acid, etc.. Are these acids useful in any way? In this article, uses of Carboxylic Acids, let’s understand the various applications of carboxylic acids in various sectors, such as methanoic acid, ethanoic acid, benzoic acid, and so on. Carboxylic acid is used in cosmetics, polymers, and medications, among other things.
Carboxylic Acid: Details
Carbon compounds that contain carboxyl group \(( – {\rm{COOH}})\) are called carboxylic acids. The carboxyl group contains a carbonyl group \(( > {\rm{C}} = {\rm{O}})\) attached to a hydroxyl group \(( – {\rm{OH}})\), and hence its name is carboxyl.
Carboxylic acids may be classified as aliphatic or aromatic depending upon the nature of the group to which the \( – {\rm{COOH}}\) group is attached. When the \( – {\rm{COOH}}\) group is attached to an H atom or to an alkyl group, the carboxylic acid is called an aliphatic carboxylic acid. They are represented by the general formula \({\rm{RCOOH}}\).
Example: Methanoic acid (Formic acid) \({\rm{ – HCOOH}}\), Ethanoic acid (Acetic acid) \({\rm{ – C}}{{\rm{H}}_3}{\rm{COOH}}\), etc.
Study Acids and Bases Concept Here
When the \( – {\rm{COOH}}\) group is attached to an aryl group, the carboxylic acid is called an aromatic carboxylic acid. They are represented by the general formula \({\rm{ ArCOOH }}\).
Example: Benzoic acid \( – {{\rm{C}}_6}{{\rm{H}}_5}{\rm{COOH}}\), toluic acid \( – {\rm{C}}{{\rm{H}}_3}{{\rm{C}}_6}{{\rm{H}}_4}{\rm{COOH}}\), etc.
Carboxylic Acid: Sources
Long chains of carboxylic acid are called fatty acids, and these are found in a variety of plants and animals. Formic acid is found in ants’ stings. Citric acid is found in citrus fruits, tamarind contains tartaric acid, malic acid is found in green apples, lactic acid is found in curd, etc.
Carboxylic acid: Uses
The various uses of common carboxylic acid are explained below:
Methanoic acid: Uses
Methanoic acid is commonly called formic acid. Its chemical formula is \({\rm{HCOOH}}\). Some of the uses of methanoic acids are,
- 1. It is used in leather tanning.
- 2. It is used as a coagulating agent for the rubber latex in the rubber industry.
- 3. It is used in textile dyeing and finishing.
- 4. It is used as an antiseptic.
- 5. It is used in medicine for the treatment of gout.
- 6. Nickel methanoate is used as a catalyst in the hydrogenation of vegetable oils.
Ethanoic acid: Uses
Ethanoic acid is commonly called acetic acid. Its chemical formula is \({\rm{C}}{{\rm{H}}_3}{\rm{COOH}}\). Some of the uses of ethanoic acids are,
- 1. Ethanoic acid is used in the manufacture of various dyes, perfumes, and rayons.
- 2. Salts of ethanoic acid are used in paints and also in certain medicines.
- 3. Ethanoates (acetates) of aluminium and chromium are used as mordants (a substance that attaches dye to the material).
- 4. It is used as a solvent.
- 5. It is used for coagulating latex.
- 6. Vinegar is the aqueous solution of \({\rm{5 – 8 \% }}\) ethanoic acid by volume. It is used in cooking and in the food industry.
Practice 10th CBSE Exam Questions
Benzoic Acid: Uses
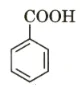
Benzoic acid is the aromatic carboxylic acid with the chemical formula \({{\rm{C}}_6}{{\rm{H}}_5}{\rm{COOH}}\). It is also written as,
The few uses of benzoic acid are:
- 1. Benzoic acid and some of its salts are used as urinary antiseptics.
- 2. Benzoic acid is used as a food preservative either in the pure form or in the form of sodium benzoate.
- 3. Benzoic acid is used in the manufacture of dyes.
- 4. Esters of benzoic acid like methyl benzoate, ethyl benzoate are used in the perfume industry.
Carboxylic Acid in Plastic
- 1. \(1,2\)-benzene dicarboxylic acids (phthalic acid) is used in the manufacture of plasticizers and resins.
- 2. \(1,4\)-benzene dicarboxylic acids (terephthalic acid) is used as basic raw material for the manufacture of polyester.
- 3. Adipic acid is used for the synthesis of nylon.
Carboxylic Acid in Cosmetics
- 1. Benzoic acid is used in lipstick and facial cleansers.
- 2. Carboxylic acids improve moisture retention in the body.
- 3. Carboxylic acid provides antioxidants and anti-aging protection to the skin.
Carboxylic Acid in Medicine
- 1. Salicylic acid is used in acne creams and in the synthesis of aspirin.
- 2. Ethanoic acid is also used in the synthesis of aspirin.
- 3. Carboxylic acids are good antimicrobial and antifungal agents.
- 4. Benzoic acid is used as a germicide and used to treat urinary tract infections.
- 5. Ascorbic acid (Vitamin \({\rm{C}}\)) helps in maintaining healthy skin, blood vessels, bones and cartilage.
Derivatives of Carboxylic Acid
Some of the derivatives of carboxylic acid are acyl halides, amides, esters, acid anhydrides, etc. Like carboxylic acid, its derivatives also have some uses. Few of them are
- 1. Acyl halides are used in the preparation of acid anhydrides, esters, and amides.
- 2. Amides are used widely in the plastic, rubber, and paper industry. It is also used in water and sewage treatment.
- 3. Acid anhydrides are used in the manufacture of explosives, pharmaceutical products, industrial chemicals, etc.
Carboxylic Acids and Esters: Uses
Esters are derivatives of a carboxylic acid obtained by replacing the hydrogen of a carboxylic acid with an alkyl or aryl group.
- 1. Due to its pleasant odour, esters are used in food flavouring, perfumes, cosmetics, essential oil, etc.
- 2. It is used as an organic solvent.
- 3. Esters are used in making surfactants.
- 4. It is used in the treatment of leprosy and asthma.
Summary of Uses of Carboxylic Acids
Carboxylic acid is an important class of organic compounds with a wide range of uses. In the article, Uses of Carboxylic Acids you have explored uses of common acids like methanoic acid, ethanoic acid and benzoic acid. You have also understood the uses of carboxylic acid in cosmetics, plastics, and medicine. Apart from carboxylic acid, derivatives of carboxylic acid also have some uses. With this topic, you can also recall the uses of derivatives of carboxylic acid.
Frequently Asked Questions on Uses of Carboxylic Acids
Frequently asked questions related to uses of carboxylic acids is listed as follows:
Q.1. What is carboxylate used for?
Ans: The conjugate base of the carboxylic acid is called a carboxylate ion. Carboxylate ions are used in the preparation of buffer solution.
Q.2. What are some carboxylic acids used in daily life?
Ans: Ethanoic acid is used in the form of vinegar in the cooking and food industry. Methanoic acid is used as an antiseptic, and it is also used in leather tanning and the textile industry.
Q.3 What are examples of carboxylic acids?
Ans: Methanoic acid \({\rm{ (HCOOH) }}\), ethanoic acid \(\left( {{\rm{C}}{{\rm{H}}_3}{\rm{COOH}}} \right)\), benzoic acid \(\left( {{{\rm{C}}_6}{{\rm{H}}_5}{\rm{COOH}}} \right)\), etc., are examples of carboxylic acid.
Q.4. Are carboxylic acids used as preservatives?
Ans: Yes, an aromatic carboxylic acid called benzoic acid is used as a food preservative.
Q.5. What are three uses of carboxylic acids?
Ans: Three uses of carboxylic acid are:
1. It is used as a coagulating agent for rubber latex in the rubber industry.
2. It is used in textile dyeing and finishing.
3. It is used as an antiseptic.
Q.6. Why are carboxylic acids important?
Ans: Carboxylic acids are important because of their wide variety of applications. These are used in the pharmaceutical industry, as food preservatives, as a solvent, etc.
Q.7. Why do carboxylic acids smell bad?
Ans: Propanoic acid to decanoic acid have a foul smell. It is due to the stale perspiration, its production by bacterial activity, etc.
Q.8. What are the sources of carboxylic acids?
Ans: Long-chain of carboxylic acids is called fatty acid. Plants and animals are the sources of it. Formic acid is found in ant’s stings. Citric acid is found in citrus fruits, tamarind contains tartaric acid, malic acid is found in green apples, lactic acid is found in curd, etc.