Oxoacids of Halogens: Preparation, Structure, Properties and Uses
Oxoacids of Halogens: Preparation, Structure, Properties and Uses: Overview
This Topic covers sub-topics such as Oxoacids of Halogens, Hypohalous Acids, Structures of Oxoacids of Chlorine, Halous Acids, Halic Acids and, Perhalic Acids
Important Questions on Oxoacids of Halogens: Preparation, Structure, Properties and Uses
What products are expected from the disproportionation reaction of hypochlorous acid?
Explain why perchloric acid is stronger than sulphuric acid?
Draw the structure of chloric acid.
What is the name of the known halous acid and draw the structure of it?
The oxidizing power of oxyacids of chlorine depend upon
Why does fluorine form only one oxoacid, . Explain.
Identify the chemical formula of oxoacid of halogen, chloric acid.
The correct order of acidity of oxoacids of chlorine is
Identify the name of oxoacid of halogen, .
Write the chemical formula for perchloric acid, hypobromous acid and fluoric acid.
Explain why fluorine does not form oxoacids?
Which oxoacid of chlorine is the most acidic?
Arrange the following :
Increasing order of thermal stability
Increasing acid strength
Increasing reducing nature
Increasing oxidation number of iodine
Increasing acid strength
Increasing oxidising power
Increasing acid strength
Increasing electronegativity
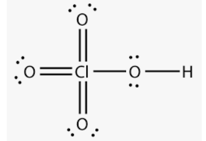
Above structure is of _____ acid.
The structure of is:
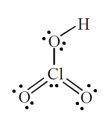
.
Which of the following oxyacid of chlorine has highest thermal stability?
Which are the different oxyacids of halogens?
The correct order of acidic strength of following halo acids is-
I.
II.
III.
Among the following, which has maximum acidic character?
The correct order of strength of oxoacids of chlorine is