A K Srivastava Solutions for Chapter: Acids, Bases and Salts, Exercise 4: Practice Questions
A K Srivastava Chemistry Solutions for Exercise - A K Srivastava Solutions for Chapter: Acids, Bases and Salts, Exercise 4: Practice Questions
Attempt the practice questions on Chapter 2: Acids, Bases and Salts, Exercise 4: Practice Questions with hints and solutions to strengthen your understanding. Science Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from A K Srivastava Solutions for Chapter: Acids, Bases and Salts, Exercise 4: Practice Questions with Hints & Solutions
State what does pH of a solution signify? Three solutions A, B and C have pH values of and respectively. Which of the solutions is highly acidic? Which solution will turn red litmus blue?
Why is an aqueous solution of sodium chloride neutral but an aqueous solution of sodium carbonate basic?
How concentrated sulphuric acid can be diluted? Describe the process.
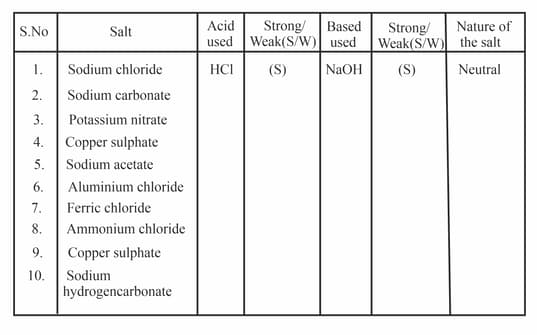
Complete the following table with the acids and bases used to form particular salt. Also given the idea of whether the acid/ base is strong or weak and nature of the salt.