A K Srivastava Solutions for Chapter: Periodic Classification of Elements, Exercise 1: ASSESSTMENT
A K Srivastava Chemistry Solutions for Exercise - A K Srivastava Solutions for Chapter: Periodic Classification of Elements, Exercise 1: ASSESSTMENT
Attempt the practice questions on Chapter 5: Periodic Classification of Elements, Exercise 1: ASSESSTMENT with hints and solutions to strengthen your understanding. Science Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from A K Srivastava Solutions for Chapter: Periodic Classification of Elements, Exercise 1: ASSESSTMENT with Hints & Solutions
Mendeleev included the noble gas elements in his periodic table.
An element with an electronic configuration is a group element.
Valency changes down the group.
Along a period, the acidic character of the oxides of elements increases and their basic character decreases.
The element with atomic number belongs to the third period and Group
Dobereiner could identify only three triads of elements. These are
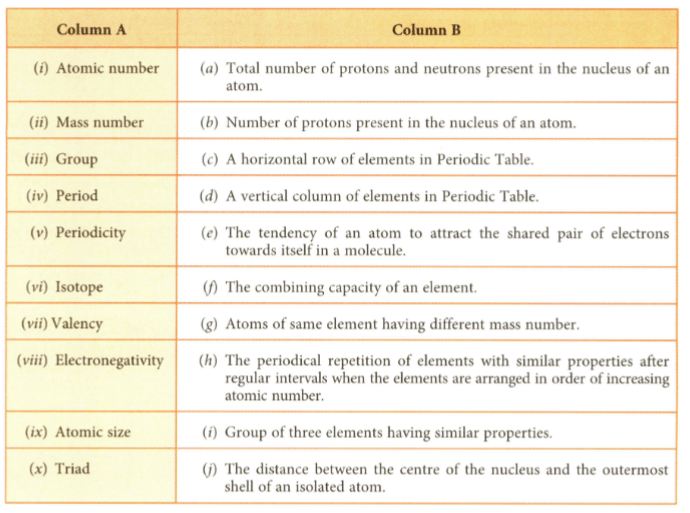
Match the vocabulary words given in Column A with their definitions given in Column B.