Saleha Pervez and Vandana Solutions for Chapter: Sample Question Paper 5, Exercise 5: SAMPLE QUESTION PAPER 5
Saleha Pervez Chemistry Solutions for Exercise - Saleha Pervez and Vandana Solutions for Chapter: Sample Question Paper 5, Exercise 5: SAMPLE QUESTION PAPER 5
Attempt the practice questions on Chapter 20: Sample Question Paper 5, Exercise 5: SAMPLE QUESTION PAPER 5 with hints and solutions to strengthen your understanding. All In One ISC Chemistry Class 12 solutions are prepared by Experienced Embibe Experts.
Questions from Saleha Pervez and Vandana Solutions for Chapter: Sample Question Paper 5, Exercise 5: SAMPLE QUESTION PAPER 5 with Hints & Solutions
Insulin maintains the blood _____ level in human body.
. Calculate the emf of the following cell reaction at .
Nitrogen pentoxide decomposes according to the following equation, . This first order reaction was allowed to proceed at and the data given below:
| Time | |
Calculate the initial rate of reaction.
For a chemical reaction, variation in concentration versus time plot is given below :
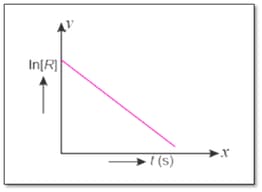
What is the order of reaction?
For a chemical reaction, variation in concentration versus time plot is given below :
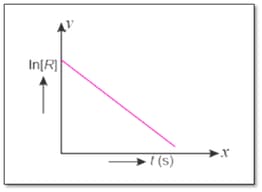
What are the units of rate constant for the reaction?
For a chemical reaction, variation in concentration versus time plot is given below :
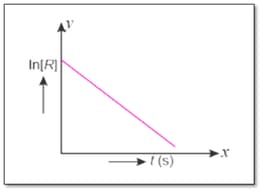
If the initial concentration of the reactant is half of the original concentration, how will change?
For a chemical reaction, variation in concentration versus time plot is given below :
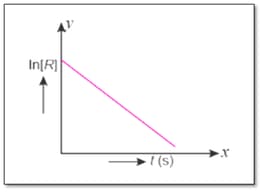
Draw the plot of log versus time .
Half-life of a first order reaction is . Then, what percentage of the initial reactant will react in ?
