Embibe Experts Solutions for Chapter: Aldehydes, Ketones and Carboxylic Acids, Exercise 4: Exercise-4
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Aldehydes, Ketones and Carboxylic Acids, Exercise 4: Exercise-4
Attempt the free practice questions on Chapter 27: Aldehydes, Ketones and Carboxylic Acids, Exercise 4: Exercise-4 with hints and solutions to strengthen your understanding. Alpha Question Bank for Engineering: Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Aldehydes, Ketones and Carboxylic Acids, Exercise 4: Exercise-4 with Hints & Solutions
Which one of the following compounds can be deprotonated by fastest?
An organic compound, does not give a precipitate with -dinitrophenylhydrazine reagent and does not react with metallic sodium. It could be:
An organic compound on treatment with acidified gives a compound , which reacts with and sodium carbonate to form tri-iodomethane. The compound is
Which of the following gives haloform reaction?
m-Chlorobenzaldehyde on reaction with conc. at room temperature gives
An organic compound , in dry benzene in the presence of anhydrous gives compound . The compound on treatment with , followed by reaction with gives compound , which on reaction with hydrazine gives a cyclised compound Identify and . Explain the formation of from .
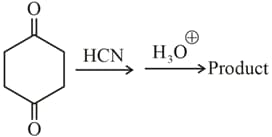
The product of the following reactions is optically inactive and exists in diastereomeric forms, report your answer as .
In which of the following reactions correct major product is mentioned?
