Embibe Experts Solutions for Chapter: Chemical Bonding and Molecular Structure, Exercise 4: Exercise-4
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Chemical Bonding and Molecular Structure, Exercise 4: Exercise-4
Attempt the free practice questions on Chapter 14: Chemical Bonding and Molecular Structure, Exercise 4: Exercise-4 with hints and solutions to strengthen your understanding. Alpha Question Bank for Engineering: Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Chemical Bonding and Molecular Structure, Exercise 4: Exercise-4 with Hints & Solutions
In , the bond length is , when is allowed to be treated with , it forms an adduct, , The bond length of in the adduct is :
The change in hybridization of aluminium when decomposes in the gas phase is :
The hybridisation of carbon atoms present in the smallest ester are:
In the following conversion, , the nitrogen atom changes its state of hybridisation from
Which one of the following has highest dipole moment?
The correct order of dipole moment for the following molecules is:
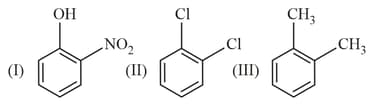
The correct order of dipole moment for the following molecules is:

The electron-pair geometry of the central oxygen atom of ozone is:
