Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 4: Exercise-4
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 4: Exercise-4
Attempt the free practice questions on Chapter 10: Chemical Kinetics, Exercise 4: Exercise-4 with hints and solutions to strengthen your understanding. Alpha Question Bank for Engineering: Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 4: Exercise-4 with Hints & Solutions
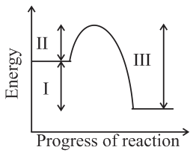
Which of the energy values marked as and in the following diagram, will change by the addition of a suitable catalyst?
Urea, decomposes at as Experimental data obtained for the reaction is given in the following plot;
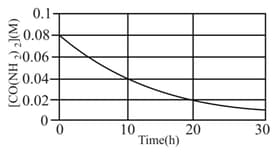
From the graph it can be inferred that-
The decomposition of can be followed by titration with and is found to be a first order reaction. The rate constant is In an experiment, the initial titre value was The titre value will be after a lapse of:
In a second order reaction, of a substance is dissociated in The time taken by of its dissociation is:
A simple mechanism for enzyme-catalyzed reaction is given by the following set of equations:
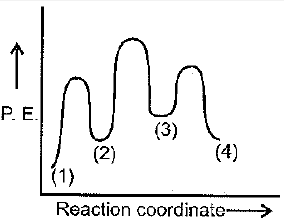
This is known as the Michaelis-Menten mechanism. The potential energy diagram is shown in the figure. Which of the following sets of identifications is correct? (Assume that the temperature and pressure are constant).
A reaction, the rate constant is expressed as The energy of the activation is:
Consider the following reactions at
(uncatalysed reaction)
(catalyst reaction)
The activation energy is lowered by the catalysed reaction. How many times is the rate of this catalysed reaction greater than that of uncatalysed reaction? (Given )
In a hypothetical reaction the activation energy for the forward and backward reaction in and respectively. The potential energy of is Then plot of vs. concentration.
