Embibe Experts Solutions for Chapter: Organic Chemistry- Some Basic Principles and Techniques, Exercise 4: Exercise-4
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Organic Chemistry- Some Basic Principles and Techniques, Exercise 4: Exercise-4
Attempt the free practice questions on Chapter 23: Organic Chemistry- Some Basic Principles and Techniques, Exercise 4: Exercise-4 with hints and solutions to strengthen your understanding. Alpha Question Bank for Engineering: Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Organic Chemistry- Some Basic Principles and Techniques, Exercise 4: Exercise-4 with Hints & Solutions
The most stable carbocation is:
Pyridine is less basic than triethylamine because:
The compound that reacts the fastest with methylamine is
Coniferyl alcohol is isolated from pine trees. The following observations were made about this alcohol :
I. It forms methylated product with Mel in presence of base.
Il. One equivalent of coniferyl alcohol reacts with two equivalents of benzoyl chloride.
III Upon ozonolysis, the coniferyl alcohol gives a product ( ).
The increasing reactivity of the sites () in the following compound is reaction is
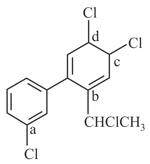
The compound which would undergo a reaction with ammonia by the mechanism is
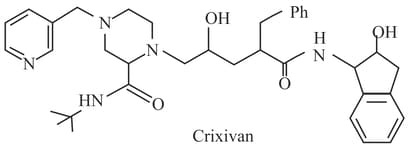
The maximum number of moles of consumed by one mole of crixivan, a drug against is
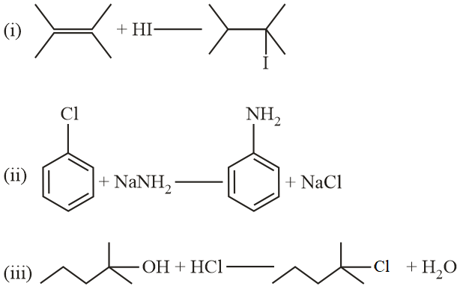
The reactions from those given below that involve a carbocation intermediate are