Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: Exercise 1
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: Exercise 1
Attempt the practice questions on Chapter 4: Thermodynamics, Exercise 1: Exercise 1 with hints and solutions to strengthen your understanding. Alpha Question Bank for Medical: Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: Exercise 1 with Hints & Solutions
In an isochoric process, the increase in internal energy is:
One mole of non-ideal gas undergoes a change of state to , with a change in internal energy . The change in enthalpy of the process in ;
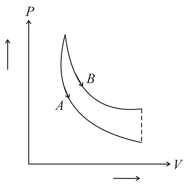
In figure, and are two adiabatic curves for two different gases. Then, and correspond to,
For the gas - phase decomposition, :
Which of the following statements is true? The entropy of the universe
Which of the following conditions, regarding a chemical process, ensures its spontaneity at all temperatures?
What is the free energy change when mole of water at and pressure is converted into steam at and pressure ?
The enthalpy change for a given reaction at is ( being positive). If the reaction occurs spontaneously at , the entropy change at that temperature
