Anil Ahlawat Solutions for Chapter: Structure of Atom, Exercise 1: EXERCISES
Anil Ahlawat Science Solutions for Exercise - Anil Ahlawat Solutions for Chapter: Structure of Atom, Exercise 1: EXERCISES
Attempt the practice questions on Chapter 4: Structure of Atom, Exercise 1: EXERCISES with hints and solutions to strengthen your understanding. NSO Science Olympiad Workbook Grade 9 solutions are prepared by Experienced Embibe Experts.
Questions from Anil Ahlawat Solutions for Chapter: Structure of Atom, Exercise 1: EXERCISES with Hints & Solutions
Which of the following statements are correct regarding the elements given below?
I The correct order of increasing proton number is
II The correct order of increasing mass number is
III There is difference in the orders of proton number and mass number.
IV The number of protons is equal to number of neutrons in all the given elements.
Hydrogen exists in three isotopic forms, respectively, known as protium, deuterium and tritium. Why are all the isotopes neutral in nature?
Identify the difference between particles of each given pair.
(I) and
(II) and
(III) and
| I | II | III | |
| (A) | Number of protons | Number of electrons | Number of neutrons |
| (B) | Number of electrons | Number of protons | Number of neutrons |
| (C) | Number of neutrons | Number of electrons | Number of protons |
| (D) | Number of protons | Number of neutrons | Number of electrons |
A compound of carbon, hydrogen and nitrogen contains these elements in the ratio . If its molecular mass is , what is the molecular formula?
Which of the given pairs of atoms contain(s) the same number of neutrons?
I and
II and
III and
IV and
Description of a few atoms is given in the table.
| Atom | ||||
| No. of protons | ||||
| No. of neutrons | ||||
| No. of electrons |
Identify a cation, an anion and a pair of isotopes from the given table:
| Cation | Anion | Pair of isotopes | |
| (A) | |||
| (B) | |||
| (C) | |||
| (D) |
Study the given mass spectrum of magnesium carefully.
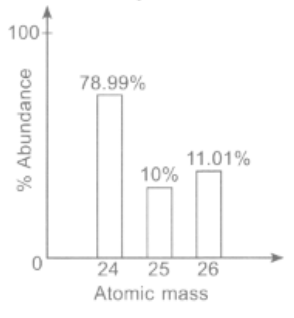
The number of protons in , number of neutrons in , and the relative atomic mass of are respectively
Electron distribution of two elements and in their outermost shell is shown below.
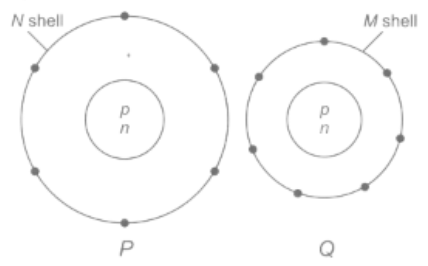
Atomic numbers of and are respectively
