Annie Termaat and Christopher Talbot Solutions for Chapter: How Can Our Energy Resources Be Accessed Fairly?, Exercise 25: SOME SUMMATIVE PROBLEMS TO TRY
Annie Termaat Chemistry Solutions for Exercise - Annie Termaat and Christopher Talbot Solutions for Chapter: How Can Our Energy Resources Be Accessed Fairly?, Exercise 25: SOME SUMMATIVE PROBLEMS TO TRY
Attempt the free practice questions on Chapter 10: How Can Our Energy Resources Be Accessed Fairly?, Exercise 25: SOME SUMMATIVE PROBLEMS TO TRY with hints and solutions to strengthen your understanding. MYP By Concept 4&5 Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Annie Termaat and Christopher Talbot Solutions for Chapter: How Can Our Energy Resources Be Accessed Fairly?, Exercise 25: SOME SUMMATIVE PROBLEMS TO TRY with Hints & Solutions
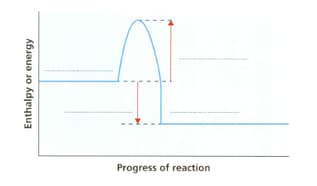
State the information required to complete the energy level diagram for a combustion reaction.
Calculate the amount of thermal energy required to raise the temperature of water in these situations. The specific heat capacity of water is .
of water from
Calculate the amount of thermal energy required to raise the temperature of water in these situations. The specific heat capacity of water is .
of water from
Iron and sulphur react together according to the following thermochemical equation:
Analyse this equation and estimate signs for and in standard conditions.
Iron and sulphur react together according to the following thermochemical equation:
Suggest whether the equation above enables you to estimate the rate of reaction.
The substances methoxy methane and ethanol both have the same molecular formula but they are structural isomers. Explain why different amounts of thermal energy are released when one mole of each substance is combusted.
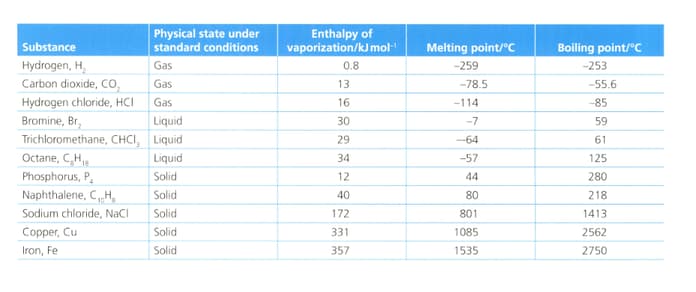
Analyse and evaluate the information in the table below to explain the general relationship between the three physical properties.
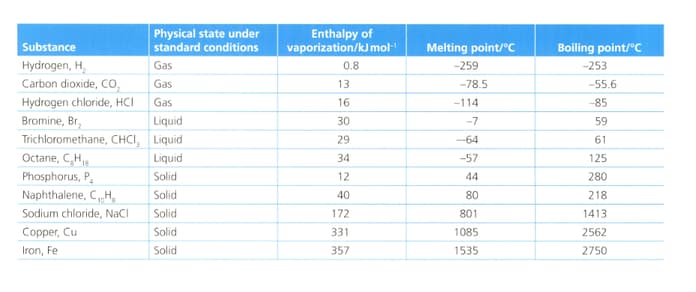
Analyse and evaluate the information in the table below to explain why the substances listed can be divided roughly into three types based on the forces between the particles in each substance.