Annie Termaat and Christopher Talbot Solutions for Chapter: How Do We Use Matter?, Exercise 23: SOME SUMMATIVE PROBLEMS TO TRY
Annie Termaat Chemistry Solutions for Exercise - Annie Termaat and Christopher Talbot Solutions for Chapter: How Do We Use Matter?, Exercise 23: SOME SUMMATIVE PROBLEMS TO TRY
Attempt the practice questions on Chapter 2: How Do We Use Matter?, Exercise 23: SOME SUMMATIVE PROBLEMS TO TRY with hints and solutions to strengthen your understanding. MYP By Concept 4&5 Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Annie Termaat and Christopher Talbot Solutions for Chapter: How Do We Use Matter?, Exercise 23: SOME SUMMATIVE PROBLEMS TO TRY with Hints & Solutions
A student decided to investigate the composition of soil in her garden by shaking a small sample in a jar of water. After a few days, most of the material in the jar had settled at the bottom, but the water above the soil had a cloudy appearance.
Interpret this information to make judgements about the density of the particles in the liquid layer and the particles in the precipitate at the bottom.
During their use perfumes often present a range of impressions. After a first fleeting scent, there can be a range of different lingering impressions. Make a scientifically supported judgement to explain how this is possible.
At a party in a bucket of iced water(i.e.containing both liquid and solid phase of water) you notice that cans of a regular soda sink, but cans of diet soda float. In the morning when all the ice cubes have melted the regular Coca-Cola and regular Dr Pepper drinks both float along with the diet sodas. Interpret this information and make scientifically supported judgements.
Explain why the values for any set of specific conditions used in chromatography are constant.
In the French perfumery industry pomade is made by placing fresh lavender on highly purified blocks of animal fat in cool dark rooms. After a few days, the fat is dissolved using cool alcohol leaving the essential oils from the flowers behind. Explain how this separation process demonstrates the awareness of the physical properties of essential oils
A plant physiologist investigating the structure of chloroplasts blended some leaves in a watery solution. Explain the equipment he would use to isolate the oil-soluble components from the mixture of blended leaves.
The table below classifies the phases of a colloid.
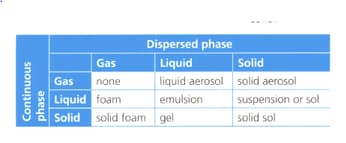
Suggest how would you use this table to classify the following materials: glass, mist, styrofoam, jelly, pumice stone, fog, whipped cream, salad dressing, paint, ink, and smoke.
Explain why there are no examples of purely gaseous colloids?
