Arun Syamal Solutions for Chapter: Matter in Our Surroundings, Exercise 3: Practice Questions
Arun Syamal Chemistry Solutions for Exercise - Arun Syamal Solutions for Chapter: Matter in Our Surroundings, Exercise 3: Practice Questions
Attempt the free practice questions on Chapter 1: Matter in Our Surroundings, Exercise 3: Practice Questions with hints and solutions to strengthen your understanding. LIVING SCIENCE CHEMISTRY 9 solutions are prepared by Experienced Embibe Experts.
Questions from Arun Syamal Solutions for Chapter: Matter in Our Surroundings, Exercise 3: Practice Questions with Hints & Solutions
Justify why water is a liquid and wood is a solid?
What are the characteristics of the particles of matter?
Suggest an experiment to demonstrate the compressibility of gases and liquids.
Draw the vibratory motions of the carbon dioxide molecule.
In the given figure, the inter conversions of matter have been given. The processes involved in the
inter conversions have been marked as A, B, C, D, E and F.
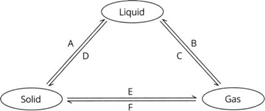
a. Name the processes marked as A, B, C, D, E and F.
b. On cooling a liquid, it changes to a new state. Name the state.
c. When a liquid is heated, it changes to a new state. Name the state.
d. When temperature is lowered and the pressure of a gas is increased, what change is expected?
While determining the boiling point of water in the laboratory, the beaker containing hot water accidentally fell on the hand of a student. A fellow student immediately put cold water on his hand and then placed ice cubes on his hand. He then took the student to the school dispensary for giving him first aid.
A. Define boiling point.
B. Why does temperature remain constant during boiling?
C. How does boiling differ from evaporation?
D. State the values displayed by the fellow student.
A student was given one packet containing white crystals of sodium chloride and another packet containing white crystals of ammonium chloride for carrying out a project. But he forgot to label the packets. One of his friends suggested him to carry out sublimation on each sample and to identify the sublimable solid. He then helped the student to set up the apparatus for carrying out sublimation and then jointly carried out sublimation of each sample separately.
A. Define sublimation.
B. What are sublimable solids? Give two examples of sublimable solids.
C. State the values demonstrated by the fellow student in helping his friend to identify the samples.
While organising the bottles containing various chemicals in the reagent racks, a student accidentally spilt a bottle of ammonia on the floor of the laboratory. The whole laboratory was soon filled with the pungent irritating odour of ammonia and white fumes were formed in the laboratory. A fellow student immediately opened the doors and windows and switched on the exhaust fans. The teacher asked all the students to move out of the laboratory in a line and then to wash their eyes with water.
A. State the name of the phenomenon which led the pungent odour of ammonia to spread in the whole laboratory.
B. Write the composition of white fumes.
C. State the values demonstrated by the fellow student and teacher in the above accident.
D. Suggest any precaution which is needed in the laboratory.
