Multiple choice questions
Arun Syamal Chemistry Solutions for Exercise - Multiple choice questions
Simple step-by-step solutions to Multiple choice questions questions of Multiple Choice Questions from LIVING SCIENCE CHEMISTRY 9. Also get 3D topic explainers, cheat sheets, and unlimited doubts solving on EMBIBE.
Questions from Multiple choice questions with Hints & Solutions
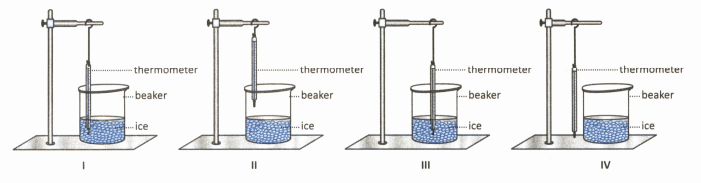
Which of the following experimental arrangements is correct to determine the melting point of ice?
While determining the boiling point of water, the teacher suggested to add some pumice stone pieces to the hard glass test tube containing water. This was done to:
The following precautions were listed for the experiment to determine the melting point of ice. The incorrect precaution is:
To determine the melting point of ice, a student immersed the thermometer bulb in crushed ice in a beaker and heated the beaker on a low flame. He would observe:
A student takes some water in a beaker and heats it over a flame for determining its boiling point. He keeps on taking its temperature readings. He would observe that the temperature of water:
When we add a solution of to an aqueous solution of , What is not observed?
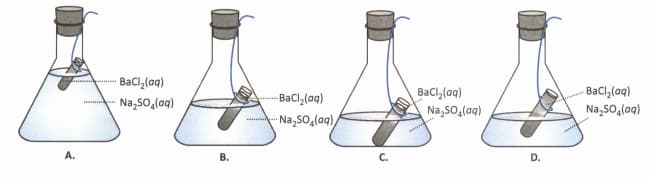
At the start of the experiment, to verify the law of conservation of mass in the laboratory, which of the following is the correct experimental set-up?
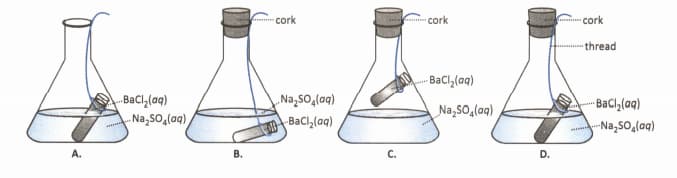
Experimental set-ups containing different volumes of the reactants , and are shown below. Which figure shows the correct volume of the reactants?