CONCEPT APPLICATION EXERCISE
B M Sharma Physics Solutions for Exercise - CONCEPT APPLICATION EXERCISE
Simple step-by-step solutions to CONCEPT APPLICATION EXERCISE questions of Kinetic Theory of Gases from PHYSICS FOR JOINT ENTRANCE EXAMINATION WAVES AND THERMODYNAMICS. Also get 3D topic explainers, cheat sheets, and unlimited doubts solving on EMBIBE.
Questions from CONCEPT APPLICATION EXERCISE with Hints & Solutions
Draw the and diagrams for an isobaric process of expansion, corresponding to moles of an ideal gas at a pressure , from to .
Aflask of volume litres, provided with a stopcock contains oxygen at and atmospheric pressure. The system is heated to a temperature of , with the stopcock open to the atmosphere. The stopcock is then closed and the flask is then cooled to its original temperature.
(a) What is the final pressure of oxygen in the flask?
(b) How many grams of oxygen remains in the flask?
An ideal gas is taken in a cylinder at pressure , volume and temperature . It is taken an isochoric process till its pressure becomes . It is now isothermally expanded to get the original pressure. Finally, the gas is isobarically compressed to its original volume . Show the process on a diagram.
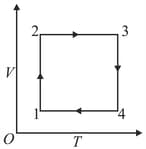
A gas has been subjected to an isothermal-isochoric cycle (see figure). Represent the same cycle on and diagrams.
An ideal gas is taken in a cylinder at pressure , volume and temperature . It is taken an isochoric process till its pressure becomes . It is now isothermally expanded to get the original pressure. Finally, the gas is isobarically compressed to its original volume . What is the temperature in the isothermal part of the process?
An ideal gas is taken in a cylinder at pressure , volume and temperature . It is taken an isochoric process till its pressure becomes . It is now isothermally expanded to get the original pressure. Finally, the gas is isobarically compressed to its original volume . What is the volume at the end of the isothermal part of the process?
A mixture of hydrogen and oxygen has volume , temperature , pressure and mass . Calculate the masses of hydrogen and oxygen in the mixture.
A container contains of hydrogen and of oxygen at ATP (pressure at and temperature ). Chemical reaction is induced by passing electric spark in the vessel till one of the gases is consumed. The temperature is brought back to its starting value . Find the pressure in the container.
