B M Sharma Solutions for Chapter: Kinetic Theory of Gases, Exercise 3: Exercises
B M Sharma Physics Solutions for Exercise - B M Sharma Solutions for Chapter: Kinetic Theory of Gases, Exercise 3: Exercises
Attempt the free practice questions on Chapter 3: Kinetic Theory of Gases, Exercise 3: Exercises with hints and solutions to strengthen your understanding. PHYSICS FOR JOINT ENTRANCE EXAMINATION WAVES AND THERMODYNAMICS solutions are prepared by Experienced Embibe Experts.
Questions from B M Sharma Solutions for Chapter: Kinetic Theory of Gases, Exercise 3: Exercises with Hints & Solutions
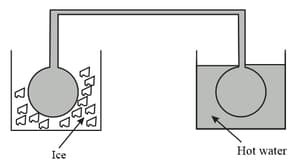
Two identical glass bulbs are interconnected by a thin glass tube at . A gas is filled in these bulbs. If one bulb is placed in ice and another bulb is placed in hot bath, the pressure of the gas become times. The temperature of hot bath will be.
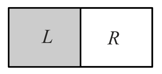
A vessel is partitioned in two equal halves by a fixed diathermic separator. Two different ideal gases are filled in left and right halves. The root-mean-square speed of the molecules in part is equal to the mean speed of molecules in the part. Then the ratio of the mass of a molecule in part to that of a molecule in part is:-
If of at is mixed with of at , the temperature of the mixture is
A closed vessel contains a mixture of two diatomic gases and . Molar mass of is times that of and mass of gas contained in the vessel is times that of . Choose the correct statement().
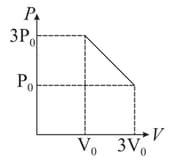
moles of a gas in a cylinder under a piston are transferred to infinitely slowly from a state with a volume of and a pressure atoms to state with and a pressure as shown in figure. If the maximum temperature that the gas will reach in this process is , what is the value of
An ideal gas has a volume at . It is confined by a spring-loaded piston in a vertical cylinder. Initially, the spring is in relaxed state. If the gas is heated to a final state of and pressure , find the work done (in ) on the spring (atmospheric pressure, ).
Oxygen is filled in a closed metal jar of volume at a pressure of and temperature . The jar has a small leak in it. The atmospheric pressure is and the atmospheric temperature is . Find the mass (in ) of the gas that leaks out by the time the pressure and the temperature inside the jar equalise with the surrounding.
Figure shows a vertical cylindrical vessel separated in two parts by a frictionless piston free to move along the length of the vessel. The length of the cylinder is and the piston divides the cylinder in the ratio of . Each of the two parts of the vessel contains mole of an ideal gas. The temperature of the gas is in each part. Calculate the mass of the piston. (in )