Embibe Experts Solutions for Chapter: Aldehydes, Ketones and Carboxylic Acids, Exercise 4: EXERCISE-4
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Aldehydes, Ketones and Carboxylic Acids, Exercise 4: EXERCISE-4
Attempt the free practice questions on Chapter 27: Aldehydes, Ketones and Carboxylic Acids, Exercise 4: EXERCISE-4 with hints and solutions to strengthen your understanding. Beta Question Bank for Engineering: Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Aldehydes, Ketones and Carboxylic Acids, Exercise 4: EXERCISE-4 with Hints & Solutions
The most stable intermediatry carbocation and major product in following reactions:
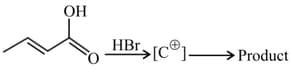
Suggest the product of the reactions:
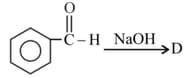
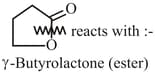
What are the product in each case?
In case of aldehydes and ketones there is addition of nucleophile but in case of acyl compound there is nucleophilic substitution. Explain.
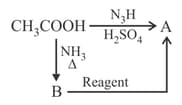
What are
Which reagent will convert into
What happens when ?
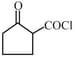 is reduced by
is reduced by
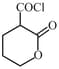 is reduced by using and by using Lindlar's catalyst.
is reduced by using and by using Lindlar's catalyst.
Complete the following sequence of reactions:
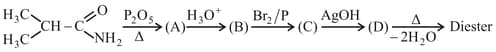
Compound having M.F. on treatment with gives and . and rearrange to give and , respectively on treatment with acid. Compounds and are all isomers of molecular formula . When is boiled with alcoholic , an oil separated out. reacts rapidly with to give back . On the other hand, on boiling with alkali followed by acidification gives a white solid , . Identify the compounds to .
