Embibe Experts Solutions for Chapter: Aldehydes, Ketones and Carboxylic Acids, Exercise 2: EXERCISE-I (Conceptual Questions)
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Aldehydes, Ketones and Carboxylic Acids, Exercise 2: EXERCISE-I (Conceptual Questions)
Attempt the practice questions on Chapter 25: Aldehydes, Ketones and Carboxylic Acids, Exercise 2: EXERCISE-I (Conceptual Questions) with hints and solutions to strengthen your understanding. Beta Question Bank for Medical: Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Aldehydes, Ketones and Carboxylic Acids, Exercise 2: EXERCISE-I (Conceptual Questions) with Hints & Solutions
The reagent used for the separation of acetaldehyde from acetophenone is:
Melting points are normally the highest for
In this reaction, , an asymmetric centre is generated. The acid obtained would be:
Acetic acid exists as dimer in benzene due to
Which one of the following, on treatment with aq. , yields the corresponding alcohol and acid:
Acetophenone when reacted with a base , yields a stable compound which has the structure :
In a set of reactions, ethyl benzene yielded a product
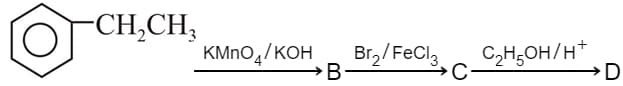
Would be :-
An organic compound , having the molecular formula , yields phenyl hydrazone and gives a negative response to the iodoform and the Tollen's test. It produces pentane on reduction. could be
