Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 3: BEGINNER'S BOX - 3
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 3: BEGINNER'S BOX - 3
Attempt the practice questions on Chapter 17: Thermodynamics, Exercise 3: BEGINNER'S BOX - 3 with hints and solutions to strengthen your understanding. Beta Question Bank for Medical: Physics solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 3: BEGINNER'S BOX - 3 with Hints & Solutions
One mole of an ideal monoatomic gas is taken around the cyclic process as shown in figure. Calculate,
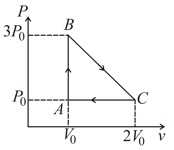
(a) the work done by the gas.
(b) the heat rejected by the gas in the path and heat absorbed in the path .
(c) the net heat absorbed by the gas in the path .
Calculate the heat supplied in figure shown for complete cycle.
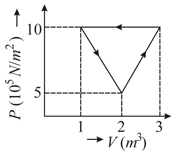
Given figure shows a P-V graph of the thermodynamic behaviour of an ideal gas. Find out work in the processes from this graph.
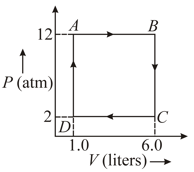
A gas is taken through a cyclic process ABCA as shown in figure. If of heat is given in the process. Calculate the value of .
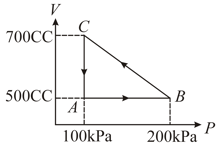
At 8 atm pressure a monoatomic gas expands from 2 litre to 5 litre then calculate the following
(a) Work done by the gas
(b) Increase in internal energy
(c) Amount of heat supplied
