Richard Harwood and Ian Lodge Solutions for Chapter: Industrial Inorganic Chemistry, Exercise 3: Exercise 9.3
Richard Harwood Chemistry Solutions for Exercise - Richard Harwood and Ian Lodge Solutions for Chapter: Industrial Inorganic Chemistry, Exercise 3: Exercise 9.3
Attempt the practice questions on Chapter 9: Industrial Inorganic Chemistry, Exercise 3: Exercise 9.3 with hints and solutions to strengthen your understanding. Cambridge IGCSE Chemistry Workbook 4th Edition solutions are prepared by Experienced Embibe Experts.
Questions from Richard Harwood and Ian Lodge Solutions for Chapter: Industrial Inorganic Chemistry, Exercise 3: Exercise 9.3 with Hints & Solutions
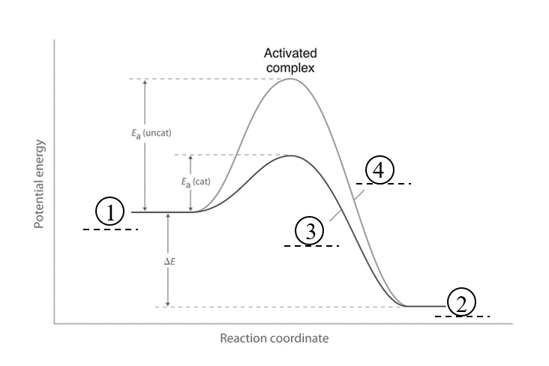
It is an energy profile diagram to show both the catalysed and the uncatalysed reaction. Label the diagram to show the following key features: the reactants and products and the catalysed and uncatalysed reactions.
The advantage of using a low temperature is the large percentage of ammonia formed. What is the disadvantage of using a low temperature?
Suggest two advantages of using high pressure in the manufacture of ammonia.
The most important use of ammonia is in fertiliser production. Fertilisers are added to the soil to improve crop yields. A farmer has the choice of two fertilisers, ammonium nitrate or diammonium hydrogen phosphate .
Show by calculation which of these fertilisers contains the greater percentage of nitrogen by mass.
State one major problem caused when the nitrates from fertilisers leach from the soil into streams and rivers.
