Richard Harwood and Ian Lodge Solutions for Chapter: Petrochemicals and Polymers, Exercise 2: Exercise 11.2
Richard Harwood Chemistry Solutions for Exercise - Richard Harwood and Ian Lodge Solutions for Chapter: Petrochemicals and Polymers, Exercise 2: Exercise 11.2
Attempt the practice questions on Chapter 11: Petrochemicals and Polymers, Exercise 2: Exercise 11.2 with hints and solutions to strengthen your understanding. Cambridge IGCSE Chemistry Workbook 4th Edition solutions are prepared by Experienced Embibe Experts.
Questions from Richard Harwood and Ian Lodge Solutions for Chapter: Petrochemicals and Polymers, Exercise 2: Exercise 11.2 with Hints & Solutions
Draw the structure of poly(ethene) showing at least two repeat units.
The structure given below is that of an addition polymerisation.
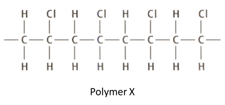
Draw the structure of the monomer from which polymer is formed.
Polymer is non-biodegradable. Describe one pollution problem that this causes.
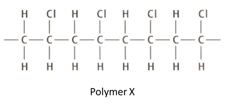
Polymer can be disposed of by burning at high temperatures. However, this can produce toxic waste gases such as hydrogen chloride. Hydrogen chloride can be removed form the waste gases by the reaction with moist calcium carbonate powder. Name the three products of this reaction.