Aiden Gill, Heidi Foxford and, Dorothy Warren Solutions for Chapter: The Periodic Table, Exercise 2: Exercise 2
Aiden Gill Science Solutions for Exercise - Aiden Gill, Heidi Foxford and, Dorothy Warren Solutions for Chapter: The Periodic Table, Exercise 2: Exercise 2
Attempt the practice questions on Chapter 5: The Periodic Table, Exercise 2: Exercise 2 with hints and solutions to strengthen your understanding. Cambridge Lower Secondary Science Stage 9: Workbook solutions are prepared by Experienced Embibe Experts.
Questions from Aiden Gill, Heidi Foxford and, Dorothy Warren Solutions for Chapter: The Periodic Table, Exercise 2: Exercise 2 with Hints & Solutions
Neon is in Group of the Periodic Table. Argon is below neon in the group. Which of these statements is true?
Read the following information about three Group elements.
At room temperature:
• bromine is a brown liquid
• iodine is a purple-black solid
• chlorine is a green gas.
Write down in order of increasing atomic number these three Group elements. Give a reason for your answer.
Magnesium and calcium are both in Group of the Periodic Table. Magnesium is above calcium. When magnesium is added to hydrochloric acid you can see bubbles of hydrogen gas. Predict what you will observe when calcium is added to some hydrochloric acid.
Explain why elements in the same group of the Periodic Table have similar chemical properties.
This is Mendeleev's Periodic Table from 1869.
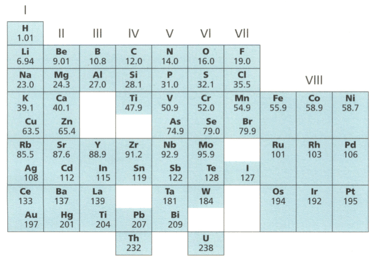
Explain why Mendeleev left gaps in his version of the Periodic Table.
