Embibe Experts Solutions for Chapter: General Principles and Processes of Isolation of Elements, Exercise 1: Exercise
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: General Principles and Processes of Isolation of Elements, Exercise 1: Exercise
Attempt the free practice questions on Chapter 6: General Principles and Processes of Isolation of Elements, Exercise 1: Exercise with hints and solutions to strengthen your understanding. Chemistry Crash Course (Based on Revised Syllabus-2023) solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: General Principles and Processes of Isolation of Elements, Exercise 1: Exercise with Hints & Solutions
What is self-reduction in metallurgy. Give example.
Give a brief note on the process of smelting used in the extraction of metals.
What is the difference between smelting and roasting?
Mention the reason for not using hydrogen as a reducing agent in metallurgy.
Define pyrometallurgy.
What type of metals are extracted electrolytically?
Explain the role of in the metallurgy of containing as impurity.
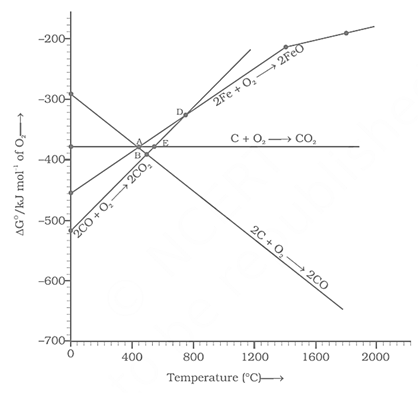
Choose the correct option of temperature at which carbon reduces to iron and produces .