Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: Exercise 1
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: Exercise 1
Attempt the practice questions on Chapter 6: Thermodynamics, Exercise 1: Exercise 1 with hints and solutions to strengthen your understanding. Chemistry Crash Course JEE Advanced solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: Exercise 1 with Hints & Solutions
One mole of , initially at a temperature of and under a pressure of is expanded adiabatically to in such a way that the temperature of the gas falls to (just above its normal boiling point). of is and is constant over the required temperature range. is supposed to behave ideally. Choose the correct options :
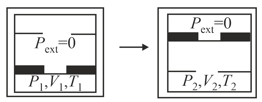
An ideal gas in thermally insulated container, at internal pressure , volume and absolute temperature expands irreversibly against zero external pressure so that the pressure of gas becomes , volume and temperature . Which of the following statement(s) is/are correct ?
A normal boiling point of a liquid is and is Assume that is independent from temperature and pressure. The correct statement (s) is/are
Read the following statements carefully and pick the correct statements:
A reaction attain equilibrium state under standard condition, then
One mole of a gas changed from its initial state to final state reversibly. If this change can be represented by a straight line in curve maximum temperature (approximate), the gas attained is '' . Then find the value of ''. (''value is in hundreds)
The enthalpy of tetramerization of in gas phase is at . The enthalpy of vaporisation for liquid and are respectively and respectively. for tetramerization of in liquid phase is at . What is the at for tetramerization reaction of in liquid phase in ?
Report the value of by rounding off to two significant figures.
In a constant volume calorimeter, of a gas(hydrocarbon) with molecular weight was burnt in excess oxygen at . The temperature of the calorimeter was found to increase from to due to the combustion process. Given that the heat capacity of the calorimeter is , If the value for the enthalpy of combustion of the gas is , Report the value of by rounding off to two significant figures,
