Competitive Thinking
Prof. Santosh Yadav Chemistry Solutions for Exercise - Competitive Thinking
Simple step-by-step solutions to Competitive Thinking questions of Halogen Derivatives of Alkanes and Arenes from MHT-CET TRIUMPH Chemistry Multiple Choice Questions Part - 2 Based on Std. XI & XII Syllabus of MHT-CET. Also get 3D topic explainers, cheat sheets, and unlimited doubts solving on EMBIBE.
Questions from Competitive Thinking with Hints & Solutions
Which of the following alkyl halide is used as a methylating agent?
of the reagent is used for dehydrohalogenation of . What will be the weight of the main product obtained?
[Atomic mass of and are and , respectively].
Assertion: Reaction of but--ene with gives -bromobutane as major product.
Reason: Addition of hydrogen halides to alkenes proceeds according to Markovnikov’s rule.
The CORRECT answer is
is treated with alcoholic . What mass of propene is obtained if yield is ?
(Atomic Mass of ).
on reaction with in the presence of peroxide forms an addition product. The number of possible stereo isomers for the product is ____.
An example of a sigma bonded organometallic compound is ____.
Which of the following reaction/s can be used for the preparation of alkyl halides?
For the following reactions:
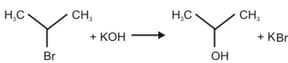
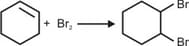
Which of the following statements is/are CORRECT?
