Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 1: Exercise-1
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 1: Exercise-1
Attempt the free practice questions on Chapter 10: Chemical Kinetics, Exercise 1: Exercise-1 with hints and solutions to strengthen your understanding. Alpha Question Bank for Engineering: Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 1: Exercise-1 with Hints & Solutions
At a gaseous reaction is found to be of first order. Starting with pure the total pressure at the end of min was of and after a long time when was completely dissociated, it was of The pressure of at the end of minutes was:
Which integrated equation is correct for the following order reaction started with only in a closed rigid vessel?
initial pressure, total pressure at time .
If no catalyst is present in acid hydrolysis of ester then rate constant is: (Where and are volumes of used to titrate reaction mixture at and )
The following data were obtained in an experiment on inversion of cane sugar; (a first order kinetics)
| Time | After a long time | ||
| Total angle of rotation (degree) |
The rate constant (in ) is
Half life of reaction: is independent of initial concentration of volume of gas after minute is at and and after completion of reaction The rate constant is-
For a hypothetical elementary reaction.
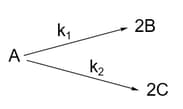
where
Initially, only moles of are present. The total number of moles of and at the end of of reaction are:
For the following parallel chain reaction 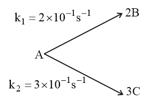 , if the sum of the concentration of and at any time is . What will be and respectively?
, if the sum of the concentration of and at any time is . What will be and respectively?
A radioactive element has a half-life of one day. After three days the amount of the element left will be:
